Mehl, J., Otto, A., Baldino, J.B. et al. The ACL-deficient knee and the prevalence of meniscus and cartilage lesions: a systematic review and meta-analysis (CRD42017076897). Arch Orthop Trauma Surg 139, 819–841 (2019). https://doi.org/10.1007/s00402-019-03128-4
The purpose of this systematic review and meta-analysis was to analyze and compare the rate of secondary meniscus and cartilage lesions diagnosed at different time points of ACL reconstruction.
A systematic search for articles comparing the rate of secondary meniscus and cartilage lesions diagnosed at different time points of ACL reconstruction was performed. PubMed central was the database used for the literature review.
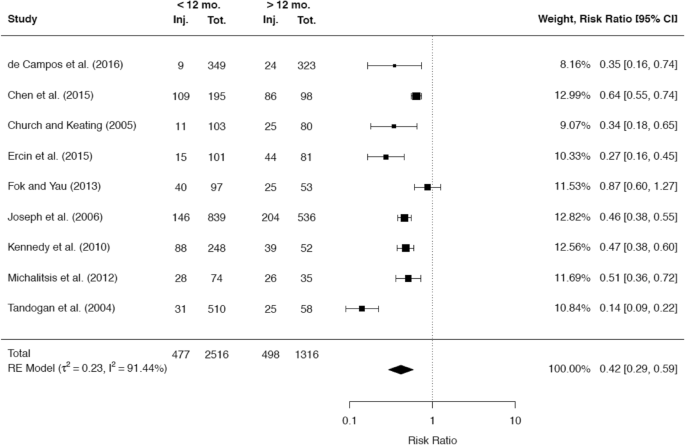
Forty articles out of 1836 were included. In 35 trials (88%), there was evidence of a positive correlation between the rate of meniscus and/or cartilage lesions and the time since ACL injury. This correlation was more evident for the medial meniscus in comparison with the lateral meniscus. In particular, a delay of more than 6 months was critical for secondary medial meniscus injuries [risk ratio 0.58 (95% CI 0.44–0.79)] and a delay of more than 12 months was critical for cartilage injuries [risk ratio 0.42 (95% CI 0.29–0.59)]. Additionally, there is evidence that the chance for meniscal repair decreases as the time since ACL rupture increases.
Chronic instability in the ACL-deficient knee is associated with a significant increase of medial meniscus injuries after 6 months followed by a significant increase of cartilage lesions after 12 months.
The anterior cruciate ligament (ACL) plays an important role in the kinematics of the knee joint. It stabilizes the tibia against anterior translation and internal rotation [67]. Especially young patients are affected by acute ruptures of the ACL which occur mostly during non-contact sports-related situations [45]. As chronic anterior rotatory instability leads to functional impairment of the patient and to the development of osteoarthritis, ACL reconstruction is generally indicated in young and active patients [52]. In Germany, the incidence of ACL reconstruction or repair is 46 per 100,000 persons/year [18].
There is evidence in the literature that the state of the menisci and the cartilage influences outcomes after ACL reconstruction [6, 16, 21]. Claes et al. [15] have shown that the rate of osteoarthritis is significantly higher in patients after ACL reconstruction and partial meniscectomy than in patients with ACL reconstruction alone, which demonstrates that the integrity of the menisci is a key factor in the development of osteoarthritis in the ACL-injured knee.
Biomechanical studies have shown that anterior tibial translation in the chronic ACL-deficient knee leads to stress concentrations on the posterior horns of the menisci [23, 53, 58]. Therefore, chronic anterior rotatory instability is considered a risk factor for the development of secondary meniscus damage after ACL rupture. However, some authors deny this conclusion [27]. In a recently published prospective randomized trial there was no significant difference in the rate of meniscus surgery between patients with acute ACL reconstruction and delayed optional ACL reconstruction [27].
The aim of this article is to perform a systematic literature review and meta-analysis of clinical trials studying the rates of meniscus and cartilage lesions observed at different time points after ACL rupture to determine if the ACL-deficient knee is a risk factor for the development of secondary meniscus and cartilage lesions.
We conducted a comprehensive literature search using the PubMed database (U.S. National Library of Medicine) to identify peer-reviewed articles about the rate of secondary meniscus and cartilage lesions with respect to the time since initial injury. This literature search was performed according to the PRISMA statement (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) [41, 47].
Before the literature search started, an open registration of the intended review was made. The study was registered at PROSPERO, which is an online, free, prospective international systematic review register (http://www.crd.york.ac.uk/PROSPERO; registry number: CRD42017076897).
Inclusion criteria to qualify for this systematic review were:
Exclusion criteria were:
Different combinations of the following keywords were utilized: anterior cruciate ligament, ACL, cartilage injury, cartilage lesion, chondral injury, chondral lesion, meniscus injury, meniscus lesion, meniscal injury, meniscal lesion. The search was conducted from October 1st till October 20th 2017 in PubMed. After identifying the articles, all references were screened for additional relevant publications. The main search was performed by reviewers JM, WP, and SS.
The publications were judged for inclusion suitability primarily from the abstract. If the abstract showed any inclusion criteria, the entire paper was read. All reviewers affirmed the inclusion of each of the final studies and performed the analysis of the articles. Any publication that did not fulfill all inclusion criteria was rejected. All reviewers had to agree to include or exclude an article. Articles, which have been included in previous systematic reviews and meta-analysis, were not excluded.
If two separate studies had the same authors and intervention but had different follow-up, then only the study with the longer follow-up was included for the outcome analysis. However, for the methodological analysis, duplicate publications, appendices of the included study, and publications of the study design were analyzed.
The quality of the included studies was analyzed by means of the Methodological Index for Non-Randomized Studies (MINORS), which consists of eight items for non-comparative studies and four additional items for comparative studies [60]. A maximum of 2 points can be assigned to each item, resulting in a maximum score of 16 points for non-comparative studies and 24 points for comparative studies. The assessment was performed independently by three reviewers (JM, AO, JB) and the average was taken as the final score.
For the analysis of further limitations, the articles were screened by each of the reviewers. If a possible limitation was found, it was noted. All limitations were discussed among the reviewers. The limitation was accepted, if all reviewers agreed.
The primary outcome parameter was the rate of meniscus lesions at different time points after ACL rupture. Secondary outcome parameters were the rate of cartilage lesions and the rate of meniscal repair at different time points after initial ACL injury.
The meta-analysis was performed using the R programming language (v3.5.1) and the metafor package (Wolfgang Viechtbauer, v2.0–0) within the RStudio-integrated development environment (v1.1.456). If the absolute numbers and ratios of patients with either meniscal injuries or cartilage injuries at different time points were given, these studies were included in separate meta-analyses. The rates of affected patients before and after the most frequently referred critical time points were compared and the risk ratios (RR) were calculated as effect size. Heterogeneity between the studies was assessed by the DerSimonian-Laird estimator for τ2 and represented by the I2 value. An I2 value of > 50% was considered to indicate substantial heterogeneity. Random-effect models were used to calculate the weighted pooled risk ratios of the included studies and forest plots were created to represent the results. Funnel plots of the included studies were assessed for asymmetry, indicating possible publication bias.
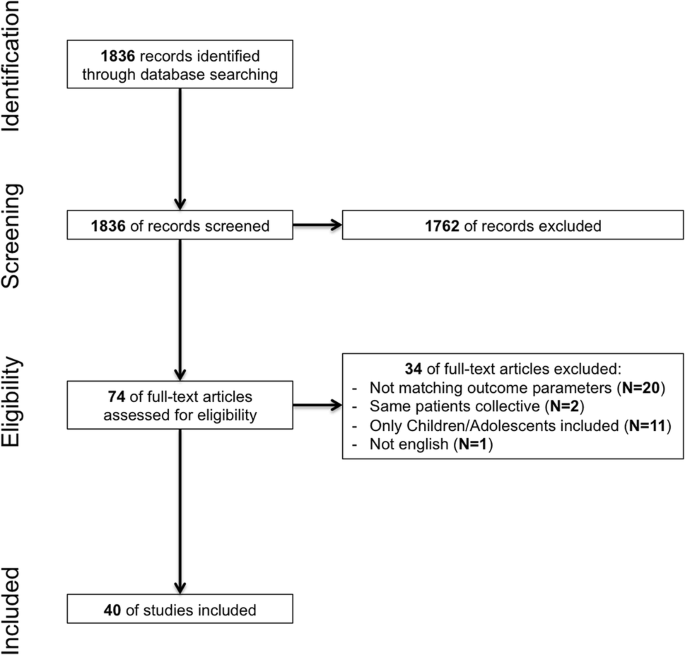
The search results are shown in Fig. 1. A total of 40 original articles were identified and included in this systematic review. Three articles published results of the same study population with three different follow-ups (1, 2, and 5 years) [25,26,27]. For the outcome analysis, only the report with the 5 years follow-up was included.
Details of the study design are shown in Table 1. Most studies were cohort studies with level III evidence. One trial was a prospective randomized trial with level I evidence [27]. The median MINORS score of the comparative studies was 17/24 (range from 12/24 to 20/24). The number of patients ranged between 31 and 5086. In most studies, the mean age of the patients was in the 3rd decade of life. Except for nine studies, the patient collectives were divided in different comparative groups according to the time from the initial injury to the ACL reconstruction. Regarding the collection of the outcome parameter, only one study referred solely to magnetic resonance imaging (MRI) [66], while all other studies referred to intraoperative findings during ACL reconstruction. In most of the included studies, the acquisition of the outcome parameter was performed at one time point. Only four studies could provide data at two different time points after the initial injury [3, 24, 63, 66]. According to the funnel plots, a publication bias could not be excluded for any of the conducted meta-analyses (Appendix, Figs. 2, 3, 4, 5, 6, 7).Table 1 Study characteristics, study quality, and summary of the main results of the 40 included studiesFull size table
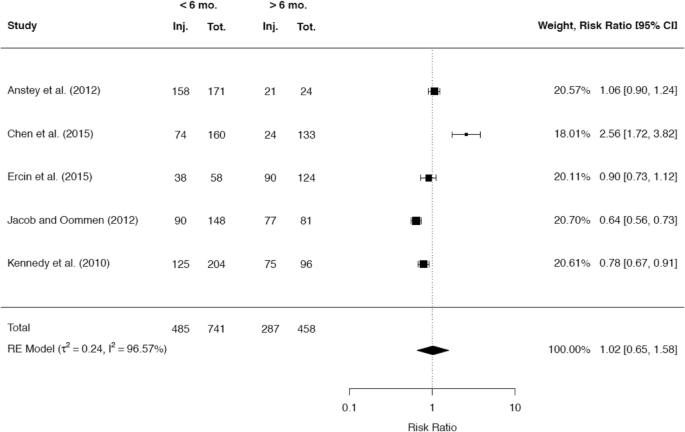
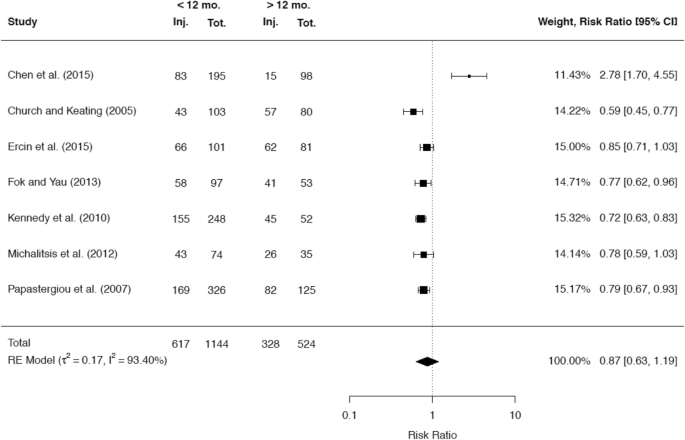
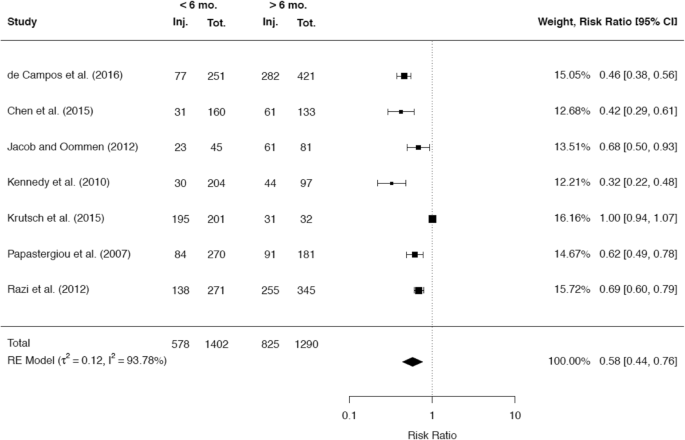
A total of 38 studies reported about the rate of meniscal injuries. Of these studies the majority of 32 studies (84.2%) showed an association between a longer time after ACL rupture and a higher rate of meniscal injuries. One study did not demonstrate this association, but found that there was a worse chance for meniscal repair with increasing time since the ACL rupture [40]. A minority of five studies did not demonstrate any correlation between time since ACL rupture and meniscal pathologies. A total of 25 studies presented cutoff values regarding the time from injury to ACLR, after which a significant increase in meniscal injuries was detected. Among these 25 studies, 10 studies reported a significant cutoff value at 6 months, while 10 studies reported a significant cutoff value at 12 months. It was possible to include the raw data of 5 and 7 studies, respectively, in separate meta-analyses to compare the rates for any meniscal injury at these two cutoff values. The results are given in Figs. 8 and 9.
Among the 32 studies that could show an association between time since injury and the rate of meniscal injuries, 23 studies made a statement about the location of the meniscal injuries. In 20 of these studies (87%), secondary meniscal damage affected only the medial meniscus and not the lateral meniscus, while in the other 3 studies (13%), both the medial and lateral menisci were affected. No study reported about secondary damages that only affected the lateral meniscus.
A total of 21 studies presented cutoff values for the time since ACL rupture, which were associated with a significant increase of medial meniscus injuries. A significant cutoff value at 6 months was stated by 8 studies and a significant cutoff value at 12 months was stated by 7 studies. It was possible to include the raw data of 7 studies each in separate meta-analyses to compare the rates for medial meniscal injuries at these two cutoff values (Figs. 10, 11).
Regarding the incidence of lateral meniscus injuries, seven studies presented cutoff values for the time since ACL rupture, which showed significant differences. While 4 studies reported a higher incidence with a longer time since the ACL rupture [2-month cutoff (n = 1), 12-month cutoff (n = 3)], 3 studies found a higher incidence with shorter time since the ACL rupture [29, 32, 51]. The stated cutoff values were relatively short in 3 of these 3 studies (6 weeks and 8 weeks), while Papastergiou et al. showed a significantly higher rate of lateral meniscal injuries with < 6 months since the ACL rupture [51].
A total of seven studies reported the rate of meniscal repair with respect to the time since the ACL rupture. All seven studies found that the rate of meniscal repair significantly decreased as the time from ACL rupture increased. The most common cutoff value that correlated with a lower chance of meniscal repair was 6 weeks (n = 3), followed by 2 months (n = 2), 3 weeks (n = 1) and 6 months (n = 1). Above these cutoff points, the rate of meniscal repair significantly declined.
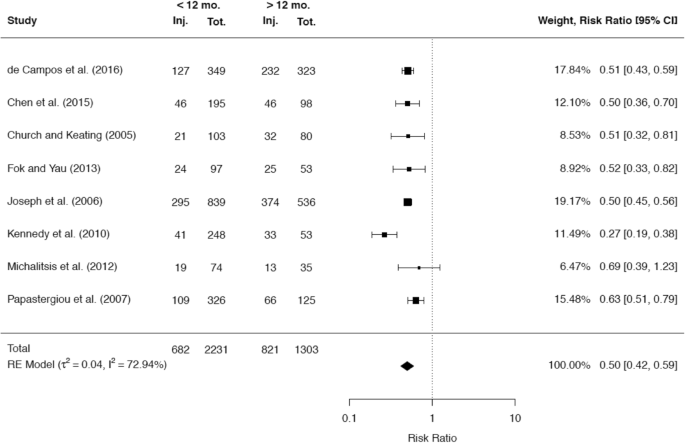
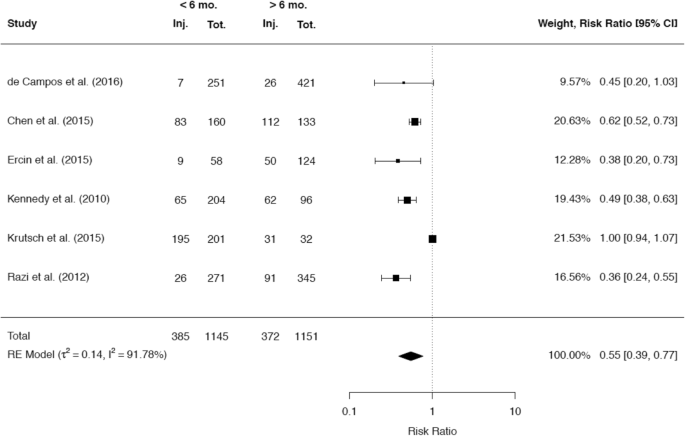
A total of 34 studies reported on the incidence of cartilage damage with respect to the time since ACL rupture. Of these studies, the majority of 29 studies (85.3%) demonstrated a positive correlation between the rate of cartilage defects in the knee joint and the time since ACL rupture. A total of 22 studies presented cutoff values, which were associated with a significantly higher incidence of cartilage injuries. The most common cutoff value of 12 months was reported by 12 studies, while a cutoff value of 6 months was stated by 5 studies. The raw data of 6 and 9 studies, respectively, were included in separate meta-analyses to compare the rates of cartilage injuries at these two cutoff values (Figs. 12, 13).
Only seven studies presented data about the location of the cartilage defects. As the subdivision of the locations varied widely among these studies, drawing specific conclusions was limited. However, the data showed that the most common site for cartilage defects was the tibiofemoral joint with a balanced distribution between the medial and lateral compartment.
A significant majority of the included studies show that chronic instability in ACL rupture leads to secondary meniscal and cartilage damage. Additionally, there is evidence that a longer time since rupture of the ACL is associated with minor chances for meniscal repair.
Only five studies did not report that time from injury was a significant factor for the presence of a meniscal lesion. However, 1 of these studies compared groups ≤ 5 weeks and > 12 weeks from ACL rupture [34] and 1 study compared groups ≤ 6 weeks and > 6 weeks from the ACL rupture [39]. Considering the results of the other included studies, these time frames might be too short to detect a significant difference. A further study which did not detect a significant association was conducted by Ahlen and Liden [1]. They compared a group of 30 patients (Group A), who received ACLR less than 5 months after injury and a group of 31 patients (Group B), who received ACLR more than 24 months after the injury. In Group A, 15 patients (50%) had any meniscal injury and in Group B 20 patients (65%). This difference did not reach statistical significance. However, looking closer at the data and comparing the rate of medial meniscus injuries separately (Group A: 4/30 patients; Group B: 14/31 patients), there was a significantly higher rate in Group B. In the study by Michalitsis et al. [46], which also did not report a significant association between time since ACL rupture and the incidence of meniscal injuries, there was a tendency towards a higher rate of meniscus lesions in the group of late ACL reconstruction after more than 12 months (acute: 22/35 patients, late: 26/35 patients). With more patients (more power), this tendency might become significant. Regarding the deleterious effect of chronic instability, it is important to note that in this study the presence of high-grade cartilage lesions was significantly increased in ACL-deficient knees when reconstruction was performed more than 12 months after injury.
A further study, which could not detect an association between the rate of meniscal injuries and the time since ACL rupture was conducted by Frobell et al. [27]. The authors performed a randomized prospective trial to compare acute ACL reconstruction with delayed optional ACL reconstruction (KANON trial). In the KANON trial, 61 patients were randomized to the group with early ACL reconstruction and 59 patients were randomized to the group with physiotherapy and delayed optional ACL reconstruction. In the group with delayed and optional ACL reconstruction ACL surgery was performed in 29 patients. There was no significant difference in the absolute amount of meniscus surgery between these two treatment groups with 29 in the group with early reconstruction and 32 in the group with initial rehabilitation and optional reconstruction. However, comparison of the two treatment groups with regard to the rate of meniscal surgery solely is misleading since this study did not distinguish between meniscal resection and refixation. Both procedures have different prognoses. In the group with early surgical treatment, almost all meniscal interventions took place in combination with the initial ACL reconstruction. It can therefore be assumed that the proportion of meniscal repair in acute ACL reconstruction is higher than in delayed ACL reconstruction. When the KANON study data are analyzed by the absolute number of meniscal interventions in the treatment groups (including second or third intervention), the results look different. In the group with early ACL reconstruction, a total of 43 meniscal surgeries were performed. In the group with delayed optional ACL reconstruction, 63 meniscal interventions were counted.
The occurrence of secondary meniscus lesions in the ACL-deficient knee can be the result of increased load in the menisci due to increased anterior tibial translation. Several biomechanical studies have shown that the load of the medial and lateral meniscus increases in the ACL-deficient knee [5, 23, 33, 58].
Regarding cartilage lesions, the included studies showed similar results to those of meniscal lesions. Only 5 of the 34 studies which reported on the incidence of cartilage lesions with respect to the time since ACL rupture did not find a significant correlation. Two of these studies compared groups with a relatively short time period after ACL rupture (≤ 3 weeks vs. > 12 weeks [34]; ≤ 6 weeks vs. > 6 weeks [38]), which may be the reason for the absence of a significant difference. The studies by Ahlen and Liden and by Arastu et al. could not detect significant differences between the acute and delayed groups, but there was a trend towards a higher incidence of cartilage lesions in the delayed groups [1, 4]. Considering the relatively small cohorts of 61 and 70 included patients, respectively, these studies might have been underpowered.
In general, the data show that after ACL rupture there is a significant increase in meniscal injuries first before the incidence of cartilage lesions rises, which supports the finding of previous studies that the integrity of the mensici is important for preventing the articular cartilage from early degeneration [15, 40].
Although some authors of the studies which are included in the present systematic review have concluded that early reconstruction preserves the knee from secondary damage, the results of their studies cannot support this conclusion for two reasons. First, because the rate of secondary lesions was in most studies assessed only once at the time of ACLR. Second, because no comparison with a control group without ACLR was performed.
However, Chalmers et al. published a systematic literature review of the long-term results after ACL reconstruction and after non-operative management to answer this research question [11]. Twenty-seven ACL reconstruction cohorts with a total of 1585 patients and 13 non-operative cohorts with a total of 685 patients were included in the review with a mean follow-up of 13.9 years. The results showed that surgical cohorts had significantly less need for subsequent meniscal surgery than non-surgical cohorts (13.9% vs. 29.4%).
The fact that chronic anterior instability leads to secondary meniscus damage and that ACL reconstruction protects the knee from secondary meniscal injury also suggests that ACL reconstruction protects from post-traumatic osteoarthritis. However, this topic is seen as contradictory in the literature. Luc et al. performed a systematic review to determine if ACL reconstruction protects the knee from osteoarthritis [42]. The authors included 38 studies in their analysis. The results showed that the only patients benefiting from ACL reconstruction were those undergoing concomitant meniscectomy. For patients with isolated ACL injury, reconstruction had no protective effect on the development of osteoarthritis. This study further underlines the association of meniscus lesions and instability in the development of osteoarthritis after ACL injury. However, Oiestad et al. showed that most studies that examined osteoarthritis after ACL rupture were of poor quality [50]. One problem with these studies was that they used different radiological scores. As a result, the results of the individual studies are not comparable. For this reason, Ajuied et al. evaluated only studies that have used the Kellgren and Lawrence classification for the analysis of radiographic osteoarthritis [2]. In this systematic review, nine studies with a minimum follow-up of 10 years were included. Six of these studies could be included in a meta-analysis. The results showed that non-surgically treated knee joints had a significantly higher risk of developing radiographic signs of OA (RR, 4.98) than knee joints undergoing ACL reconstruction (RR, 3.62). However, the results also showed that surgically treated knee joints had significantly more often high-grade OA. One possible explanation is that surgery in general may be a risk factor for developing OA changes. Additionally, it must be considered that patients who underwent ACL reconstruction often had the aim to return to sport and were therefore exposed to pivoting and cutting movements more frequently.
Another problem is that long-term results (> 10 years) are needed to evaluate the effect of a surgical procedure on the development of osteoarthritis. This implies that in these studies surgical procedures for ACL replacement were investigated which were used more than 20 years ago. A relevant point is that the transtibial drilling technique was widespread in the early days of athroscopic cruciate ligament surgery. A problem with this drilling technique is that there is a greater risk for a high non-anatomical tunnel misplacement. It can be assumed that the studies analyzed in the previous systematic reviews on posttraumatic osteoarthritis after ACL reconstruction showed a high rate of non-anatomical or partially anatomical femoral and tibial tunnel placements. Biomechanical studies have shown that this tunnel position is not suitable for restoring the rotational stability of the knee joint [8]. Clinical studies have shown that transtibial drilling technique leads to increased laxity and poorer functional scores than the medial portal drilling technique widely used today [56].
The present systematic review has some limitations. One limitation is the quality of the included studies. The majority of studies were of level III evidence according to the Oxford (UK) CEBM levels of evidence. However, this hierarchy of evidence could be questioned, because these guidelines have failed to properly weigh the merits of certain non-randomized controlled trials. One merit of a non-randomized controlled trial is a larger sample of patients and a higher statistical power. The ranking of RCTs near the top of such hierarchies has also been criticized by other authors [10, 52, 64].
A possible publication bias of the studies which were included in the meta-analyses cannot be ruled out, which has to be considered as a further limitation.
Moreover, there is a relevant heterogeneity of the included studies regarding the evaluation of cartilage defects. Different grading systems were used in different studies and some studies did not refer to any grading system. Additionally, some studies considered any kind of cartilage lesion and other studies considered only high-grade cartilage lesions.
The results of this systematic review clearly show that chronic anterior rotatory instability leads to secondary knee injuries with a significant increase of medial meniscus lesions after 6 months followed by a significant increase of cartilage lesions after 12 months. Although there is already evidence of a protective effect of ACL reconstruction in the literature, further studies are needed to confirm these results.
There is no funding source.
Correspondence to Wolf Petersen.
JM, AA, RA, ABI, SS and WP are members of the ligament committee of the German knee society (DKG). ABI receives royalties from Arthrex and consultant fees from Arthrosurface and Medi Bayreuth outside the submitted work. SS receives personal fees from Smith & Nephew, Arthrex and Conmed/Linvatec outside the submitted work. WP receives consultant fees from Karl Storz endoscopy, AAP implants and Otto Bock health care. All other authors declare that they have no conflicts of interest.
This article does not contain any studies with human participants or animals performed by any of the authors.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
See Figs. 8, 9, 10, 11, 12 and 13.