Surgical site infections (SSI’s) are a major concern as they account for a significant amount of morbidity, mortality and prolonged hospitalization [1, 2, 3, 4]. Since the discovery of microorganisms, various methods of preventing microorganisms from colonizing surgical wounds have been developed. These methods vary from surgical dressings and sterilized tools to special air management in the operating room. As the human skin contains up to one trillion commensal bacteria, it is an obvious source of SSI’s [5]. It is conceivable that elimination of microorganisms from the skin will result in a reduction of SSI.
Skin antisepsis is even more important in trauma/orthopedic procedures of the foot and ankle as in this type of surgery the rate of SSI’s is higher compared to other parts of the body [6, 7, 8, 9]. Examples are calcaneal fracture surgery with infection rates up to 33% worldwide and an average of 12.1% in Europe [10]. In ankle fractures which need open reduction and internal fixation, an infection rate of 4.3% is described [11]. In implant removal, especially on the lower extremity, SSI rates above 10% are reported [12]. The foot and ankle differ in many ways from other parts of the body by having less apocrine, but more eccrine glands, an alkaline environment, being heavily ridged, containing less pilosebaceous units (structure consisting of hair, hair follicle, arrector pili muscles, sebaceous gland) and being covered by socks and shoes most of the time creating a warm and moist environment which is ideal for growth of microorganisms [7, 13].
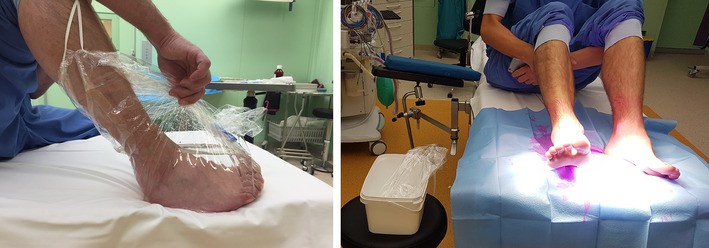
The most frequently used substances for preoperative skin cleansing are: (1) alcohols, (2) chlorhexidine (CHG), (3) povidone–iodine (PVI) or a combination of these [14]. All these substances have different qualities, some of which make them more suitable while others make them less favorable. Alcohol is microbiologically the most active; it is ten times more potent than CHG and PVI when it comes in its therapeutic concentration between 70 and 90% [14]. Chlorhexidine also has a direct effect, but has, in contrast to alcohol, a longer residual effect for up to 6 h. Furthermore it is active over a wide pH (5–8) when used within the correct therapeutic concentrations (0.5–3.5%) [7, 14, 15, 16]. PVI usually comes in an aqueous solution with its therapeutic concentration between 5 and 10% or between 0.5 and 1% ‘available’ iodine. It is active within a narrow pH (≈ 6) and needs to dry first before it becomes active [17]. Traditionally the administration of these substances is performed by painting of the surgical field. However, Ng et al. have shown a reduction in bacterial isolation after a preoperative chlorhexidine footbath [18]. Since alcohol is microbiologically active, we hypothesized an alcohol footbath could lead to an even larger reduction. To the best of our knowledge, alcohol has never been investigated as a footbath. Therefore, we combined the footbath with the common practice.
Keeping the unique properties of each substance in mind, we hypothesized that a combination of alcohol footbath with chlorhexidine would provide better preoperative skin antisepsis compared to traditional preoperative skin antisepsis. Our primary research question was: does a preoperative regime of bathing the foot and ankle in alcohol for 5 min have an additive value in bacterial load reduction. Secondary research question was: which organisms remain after preoperative skin antisepsis.