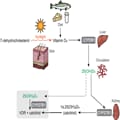
Vitamin D Deficiency and Insufficiency
Much debate has centered around the definitions of vitamin D deficiency and insufficiency. In a 2011 consensus statement, the Institute of Medicine concluded that 25(OH)D levels >20 ng/mL (50 nmol/L) were "identified as meeting the needs of at least 97.5% of the [North American] population across all life-stage groups."[23] This requirement could be met by ingestion of 600 IU of vitamin D for most adults and 800 IU for those aged >70 years. The Endocrine Society's clinical practice guideline for the evaluation, prevention, and treatment of vitamin D deficiency defined "deficiency" as 25(OH)D levels <20 ng/mL (50 nmol/L), "insufficiency" as 25(OH)D levels of 21 to 29 ng/mL (50 to 75 nmol/L), and normal levels as >30 ng/mL (75 nmol/L).[24] The Endocrine Society, however, suggested that 600 or 800 IU was not sufficient intake to ensure normal levels in most people. Therefore, they raised the recommended intake to approximately 1,500 IU cumulatively from all sources. Both of these sets of criteria were based on the estimated requirements for maintaining bone health, whereas vitamin D requirements for extraskeletal benefits were not specifically addressed. Because of these differing recommendations, no consensus agreement exists on whether 20 ng/mL or 30 ng/mL and above constitutes a so-called normal vitamin D level.
These consensus statements called specific attention to the increased rates of vitamin D deficiency in persons with dark skin and those who do not receive adequate amounts of direct sunlight, particularly at more northern latitudes. Athletes who participate in sports that mostly occur in an indoor setting may also be at risk of deficiency. A recent systematic review of vitamin D status in >2,000 athletes with a mean age of 22 years found that 56% had vitamin D inadequacy (defined as <32 ng/mL).[25] The risk increased considerably for athletes who played winter and spring season sports, those who played indoor sports, and those at higher latitudes (>40° north). Interestingly, rates of vitamin D insufficiency were substantially higher in the Middle East, possibly because of increased indoor activity in response to extreme outdoor heat, particularly during the summer months. In contrast, Wentz et al[26] investigated serum 25(OH)D levels in a cohort of distance runners in the southeastern United States. They reported that only 5% were vitamin D deficient and 13% were vitamin D insufficient. The authors of the study concluded that training outdoors in a latitude where vitamin D synthesis occurs year-round reduces the risk of vitamin D deficiency. Alternatively, because the study participants were healthy athletes with adequate sun exposure, this finding may suggest that previously defined levels of vitamin D deficiency and insufficiency do not adequately capture the vitamin D status of all persons.
Effect on Muscle Strength
Although vitamin D has been known to affect muscle histochemistry, data have also become available to demonstrate that increased vitamin D levels and supplementation can have a positive effect in otherwise healthy people. One of the first large studies to investigate this association examined nearly 1,000 patients and correlated serum 25(OH)D levels with physical activity and muscle strength.[27] Although the authors found that many study participants were vitamin D deficient, increased 25(OH)D levels were substantially correlated with a higher physical activity metric (short performance physical battery) and with greater hand grip strength. This result was found in participants above and below both the lower (50 nmol/L) and proposed higher (75 nmol/L) thresholds for serum 25(OH)D insufficiency.
These findings were corroborated in a level I meta-analysis of randomized controlled trials investigating the effect of vitamin D supplementation on muscle strength in a young and active cohort.[28] The study examined 310 participants who received either vitamin D3 or placebo. The participants had a mean baseline serum 25(OH)D of 12.3 ng/mL and an average age of 24 years. Strength metrics for the upper extremity included handheld dynamometer grip strength, one repetition maximum bench press, and assessment with isokinetic dynamometers. Lower extremity strength testing consisted of single-repetition maximum leg press, free weight squats, gastrocnemius-soleus strength isokinetic dynamometer testing, and isometric quadriceps contraction. The authors found that vitamin D supplementation demonstrated a statistically significant positive effect on both upper and lower limb strength indices. Consistent with prior literature,[29] Tomlinson et al[28] also suggested that daily vitamin D3 administration would be more effective than weekly or monthly doses at improving muscle strength.
Effect on Sports Performance
Vitamin D is not currently listed as a banned substance by the World Anti-Doping Agency. This fact has led to interest among investigators in examining the effect of vitamin D on sport-specific performance measures. In a recent study, Maroon et al[30] investigated the correlation of vitamin D levels and the ability to obtain a professional contract in a cohort of 80 American professional football players. Although 77% of the athletes studied were characterized as vitamin D "deficient" or "insufficient," a statistically significant correlation was found between lower vitamin D levels and release from the team (because of either poor performance or injury) before the start of the regular season.
In a randomized placebo-controlled trial, the effect of vitamin D (5,000 IU per day over an 8-week period) on sprint times and vertical jumps was assessed in a cohort of athletes, with nearly 70% having baseline serum 25(OH)D levels of <20 ng/mL.[31] The group receiving vitamin D supplementation recorded substantially increased vertical jump heights from the beginning to the end of the study period, whereas no change was observed in the placebo-controlled group. A different randomized controlled trial of postmenarchal women examined the effect of vitamin D supplementation on lower limb muscle force, power, velocity, jumping height, and the Esslinger Fitness Index.[32,33] The authors of the study found that jumping and movement efficiency was substantially increased in the vitamin D group, compared with the control group, a result potentially stemming from improvements in jumping velocity and height.[33]
Effect on Athletic Injury Risk
Because of the important role of vitamin D in bone health, much of the current literature has focused on the association between vitamin D levels and the risk of stress fractures in the athletically active population.[34–39]Davey et al[36] performed a prospective age-matched cohort study of >1,000 Royal Marine recruits in the United Kingdom to evaluate the association between vitamin D levels and stress fracture risk. They identified 92 stress fractures and found that recruits with a baseline serum vitamin D concentration of <50 nmol/L (20 ng/mL) had a 60% higher incidence of stress fracture than recruits with vitamin D concentrations above this threshold had. As would be expected, serum vitamin D levels were found to peak in the summer months, but no association between occurrence of fracture and the time of year could be found.[36] The results of this study were supported by a recent systematic review of eight investigations comprising >2,600 military recruits, which found an association between lower vitamin D levels and increased incidence of stress fracture.[35]
Similar results have been found in a nonmilitary population. In a retrospective review of 124 patients with imaging-confirmed stress fractures at a single center over a 3-year period, Miller et al[37] obtained vitamin D levels near the time of evaluation and reported deficient or insufficient vitamin D levels in 83% of patients. Another investigation found that vitamin D levels were considerably lower in American professional football players with at least one bone fracture, compared with players without a history of fracture.[30]
In contrast, endurance athletes who participated exclusively in an outdoor environment in the southern United States were evaluated for serum 25(OH)D levels, history of stress fracture, bone mineral density, and PTH levels.[26] The authors of that study found low levels of vitamin D deficiency and insufficiency in these athletes and no difference in any outcome measure between the athletes with insufficiency or deficiency and those with adequate levels of serum 25(OH)D.
Adverse Effects
Because vitamin D is a key regulator of calcium homeostasis and many kidney stones are calcium based, some authors have hypothesized an association between vitamin D levels and an increased risk of nephrolithiasis.[40] The evidence regarding whether vitamin D levels and/or supplementation can lead to an increased risk of kidney stones is mixed. In a Women's Health Initiative study, >36,000 postmenopausal women were randomized to receive either 500 mg calcium carbonate plus 200 IU vitamin D3 twice daily or a placebo, with the main outcome measure being the incidence of urinary tract stones during the 7-year follow-up period.[41] The authors reported that the incidence of self-reported clinically diagnosed urinary tract stones was higher in the experimental group than in the control group (hazard ratio 1.17). More recent studies, however, have not found a positive association between vitamin D and risk of nephrolithiasis. In one investigation, >2,000 patients were followed to determine the incidence of renal stones over a median of 19 months after measurement of serum 25(OH)D levels.[42] The authors of the study concluded that serum 25(OH)D levels of 20 to 100 ng/mL had no association with the formation of kidney stones. Gallagher et al[43] performed a randomized controlled investigation examining the incidence of hypercalciuria and hypercalcemia in 163 vitamin D–deficient women receiving 400 to 4,800 IU/d of vitamin D as well as calcium citrate to achieve a calcium intake of 1,200 mg/d. The authors found no relationship between hypercalciuria and hypercalcemia and vitamin D dose and reported that hypercalciuria was equally common in the treatment and placebo groups. One factor that may influence these findings is the use of calcium carbonate in the Women's Health Initiative study[41] versus the use of calcium citrate in the investigation by Gallagher et al.[43] Because citrate is an inhibitor of stone formation, some physicians advise patients who take both vitamin D and calcium supplementation to use calcium citrate rather than calcium carbonate and to maintain adequate fluid intake. However, many factors other than serum 25(OH)D levels contribute to stone formation, including body mass index, sex, genetics, and diet.
Toxicity
Vitamin D toxicity can result from the ingestion of excessive quantities of vitamin D supplements. No cases of vitamin D toxicity from sunlight or regular dietary intake have been reported. The symptoms of vitamin D toxicity center around the resultant hypercalcemia, leading to an array of symptoms, including anorexia, frequent urination, excessive thirst, nausea, vomiting, and in severe cases, altered mental status and kidney dysfunction. Many cases of vitamin D intoxication are the result of improperly manufactured supplements.[44,45] In one report, a >1,000-fold error was made in the amount of vitamin D in a supplement.[44] Two patients were affected, with both ingesting nearly 1,000,000 IU per day for >1 month, resulting in 25(OH)D levels >1,000 ng/mL. Both patients received supportive treatment with fluids and cessation of vitamin D intake. Diphosphonates were also used to decrease serum calcium levels.
Summary
Vitamin D is a secosteroid hormone that has many important functions, including the regulation of calcium homeostasis and bone metabolism. In addition, VDR has been found in muscle cells, where activation of the receptor has multiple direct effects, including enhanced movement of myosin over the actin filaments through augmentation of calcium release from the sarcoplasmic reticulum. These molecular and cellular changes, as well as other actions in muscle, may be responsible for the findings of decreased fall risk in the elderly, improved muscle strength, lower injury rates, and enhanced athletic performance associated with sufficient 25(OH)D levels. These molecular and cellular changes may even benefit people with adequate vitamin D levels, but particularly improve function and decrease fracture risk in those who are vitamin D deficient.
- Costa EM, Blau HM, Feldman D: 1,25-dihydroxyvitamin D3 receptors and hormonal responses in cloned human skeletal muscle cells. Endocrinology 1986; 119(5):2214–2220.
- Christakos S, Dhawan P, Verstuyf A, Verlinden L, Carmeliet G: Vitamin D: Metabolism, molecular mechanism of action, and pleiotropic effects. Physiol Rev 2016;96(1):365–408.
- DeLuca HF: Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr 2004;80(6 suppl):1689S-1696S.
- Boyce BF, Xiu Y, Li J, Xing L, Yao Z: NFkB-mediated regulation of osteoclastogenesis. Endocrinol Metab (Seoul) 2015;30(1):35–44.
- Gorter EA, Hamdy NA, Appelman-Dijkstra NM, Schipper IB: The role of vitamin D in human fracture healing: A systematic review of the literature. Bone 2014;64:288–297.
- Brinker MR, O'Connor DP, Monla YT, Earthman TP: Metabolic and endocrine abnormalities in patients with nonunions. J Orthop Trauma 2007;21(8):557–570.
- Boszczyk AM, Zakrzewski P, Pomianowski S: Vitamin D concentration in patients with normal and impaired bone union. Pol Orthop Traumatol 2013;78:1–3.
- Haining SA, Atkins RM, Guilland-Cumming DF, Sharrard WJ, Russell RG, Kanis JA: Vitamin D metabolites in patients with established non-union of fracture. Bone Miner 1986;1(3):205–209.
- Olsson K, Saini A, Strömberg A, et al: Evidence for vitamin D receptor expression and direct effects of 1a,25(OH)2D3 in human skeletal muscle precursor cells. Endocrinology2016;157(1):98–111.
- Pojednic RM, Ceglia L: The emerging biomolecular role of vitamin D in skeletal muscle. Exerc Sport Sci Rev 2014;42(2): 76–81.
- Endo I, Inoue D, Mitsui T, et al: Deletion of vitamin D receptor gene in mice results in abnormal skeletal muscle development with deregulated expression of myoregulatory transcription factors. Endocrinology 2003; 144(12):5138–5144.
- Irazoqui AP, Heim NB, Boland RL, Buitrago CG: 1a,25 dihydroxi-vitamin D3 modulates CDK4 and CDK6 expression and localization. Biochem Biophys Res Commun 2015;459(1):137–142.
- Irazoqui AP, Boland RL, Buitrago CG: Actions of 1,25(OH)2-vitamin D3 on the cellular cycle depend on VDR and p38 MAPK in skeletal muscle cells. J Mol Endocrinol 2014;53(3):331–343.
- Norman AW, Okamura WH, Bishop JE, Henry HL: Update on biological actions of 1alpha,25(OH)2-vitamin D3 (rapid effects) and 24R,25(OH)2-vitamin D3. Mol Cell Endocrinol2002;197(1-2):1–13.
- Girgis CM, Clifton-Bligh RJ, Hamrick MW, Holick MF, Gunton JE: The roles of vitamin D in skeletal muscle: Form, function, and metabolism. Endocr Rev 2013;34(1):33–83.
- Boland R: Role of vitamin D in skeletal muscle function. Endocr Rev 1986;7(4): 434–448.
- Tripkovic L, Lambert H, Hart K, et al: Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: A systematic review and meta-analysis. Am J Clin Nutr 2012;95(6):1357–1364.
- Logan VF, Gray AR, Peddie MC, Harper MJ, Houghton LA: Long-term vitamin D3 supplementation is more effective than vitamin D2 in maintaining serum 25-hydroxyvitamin D status over the winter months. Br J Nutr 2013;109(6):1082–1088.
- Horst R, Prapong S, Reinhardt T, Koszewski N, Knutson J, Bishop C: Comparison of the relative effects of 1,24-dihydroxyvitamin D(2) [1, 24-(OH) (2)D(2)], 1,24-dihydroxyvitamin D(3) [1,24-(OH)(2)D(3)], and 1,25-dihydroxyvitamin D(3) [1,25-(OH)(2)D (3)] on selected vitamin D-regulated events in the rat. Biochem Pharmacol 2000;60 (5):701–708.
- Powe CE, Evans MK, Wenger J, et al: Vitamin D-binding protein and vitamin D status of black Americans and white Americans. N Engl J Med 2013;369(21): 1991–2000.
- Wilson RT, Bortner JD Jr, Roff A, et al: Genetic and environmental influences on plasma vitamin D binding protein concentrations. Transl Res 2015;165(6): 667–676.
- Nielson CM, Jones KS, Chun RF, et al; Osteoporotic Fractures in Men (MrOS) Research Group: Free 25-hydroxyvitamin D: Impact of vitamin D binding protein assays on racial-genotypic associations. J Clin Endocrinol Metab 2016;101(5): 2226–2234.
- Ross AC, Manson JE, Abrams SA, et al: The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. J Clin Endocrinol Metab 2011;96 (1):53–58.
- Holick MF, Binkley NC, Bischoff-Ferrari HA, et al; Endocrine Society: Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011;96(7):1911–1930.
- Farrokhyar F, Tabasinejad R, Dao D, et al: Prevalence of vitamin D inadequacy in athletes: A systematic-review and metaanalysis. Sports Med 2015;45(3):365–378.
- Wentz LM, Liu PY, Ilich JZ, Haymes EM: Female distance runners training in southeastern United States have adequate vitamin D status. Int J Sport Nutr Exerc Metab 2016;26(5):397–403.
- Houston DK, Cesari M, Ferrucci L, et al: Association between vitamin D status and physical performance: The InCHIANTI study. J Gerontol A Biol Sci Med Sci 2007; 62(4):440–446.
- Tomlinson PB, Joseph C, Angioi M: Effects of vitamin D supplementation on upper and lower body muscle strength levels in healthy individuals: A systematic review with metaanalysis. J Sci Med Sport 2015;18(5): 575–580.
- Sanders KM, Stuart AL, Williamson EJ, et al: Annual high-dose oral vitamin D and falls and fractures in older women: A randomized controlled trial. JAMA 2010; 303(18):1815–1822.
- Maroon JC, Mathyssek CM, Bost JW, et al: Vitamin D profile in National Football League players. Am J Sports Med 2015;43 (5):1241–1245.
- Close GL, Russell J, Cobley JN, et al: Assessment of vitamin D concentration in non-supplemented professional athletes and healthy adults during the winter months in the UK: Implications for skeletal muscle function. J Sports Sci 2013;31(4): 344–353.
- Runge M, Rittweger J, Russo CR, Schiessl H, Felsenberg D: Is muscle power output a key factor in the age-related decline in physical performance? A comparison of muscle cross section, chair-rising test and jumping power. Clin Physiol Funct Imaging 2004;24(6):335–340.
- Ward KA, Das G, Roberts SA, et al: A randomized, controlled trial of vitamin D supplementation upon musculoskeletal health in postmenarchal females. J Clin Endocrinol Metab 2010;95(10): 4643–4651.
- Clutton J, Perera A: Vitamin D insufficiency and deficiency in patients with fractures of the fifth metatarsal. Foot (Edinb) 2016;27: 50–52.
- Dao D, Sodhi S, Tabasinejad R, et al: Serum 25-hydroxyvitamin D levels and stress fractures in military personnel: A systematic review and meta-analysis. Am J Sports Med2015;43(8):2064–2072.
- Davey T, Lanham-New SA, Shaw AM, et al: Low serum 25-hydroxyvitamin D is associated with increased risk of stress fracture during Royal Marine recruit training. Osteoporos Int2016;27(1):171–179.
- Miller JR, Dunn KW, Ciliberti LJ Jr, Patel RD, Swanson BA: Association of vitamin D with stress fractures: A retrospective cohort study. J Foot Ankle Surg 2016;55(1): 117–120.
- Shimasaki Y, Nagao M, Miyamori T, et al: Evaluating the risk of a fifth metatarsal stress fracture by measuring the serum 25-hydroxyvitamin D levels. Foot Ankle Int2016;37(3):307–311.
- Smith JT, Halim K, Palms DA, Okike K, Bluman EM, Chiodo CP: Prevalence of vitamin D deficiency in patients with foot and ankle injuries. Foot Ankle Int 2014;35 (1):8–13.
- 40. Ferraro PM, Taylor EN, Gambaro G, Curhan GC: Vitamin D intake and the risk of incident kidney stones. J Urol 2017;197 (2):405–410.
- Wallace RB, Wactawski-Wende J, O'Sullivan MJ, et al: Urinary tract stone occurrence in the Women's Health Initiative (WHI) randomized clinical trial of calcium and vitamin D supplements. Am J Clin Nutr 2011;94(1):270–277.
- Nguyen S, Baggerly L, French C, Heaney RP, Gorham ED, Garland CF: 25-Hydroxyvitamin D in the range of 20 to 100 ng/mL and incidence of kidney stones. Am J Public Health2014;104(9): 1783–1787.
- Gallagher JC, Smith LM, Yalamanchili V: Incidence of hypercalciuria and hypercalcemia during vitamin D and calcium supplementation in older women. Menopause2014;21(11):1173–1180.
- Araki T, Holick MF, Alfonso BD, et al: Vitamin D intoxication with severe hypercalcemia due to manufacturing and labeling errors of two dietary supplements made in the United States. J Clin Endocrinol Metab 2011;96(12): 3603–3608.
- Kara C, Gunindi F, Ustyol A, Aydin M: Vitamin D intoxication due to an erroneously manufactured dietary supplement in seven children. Pediatrics 2014;133(1):e240-e244.