Management of Carpal Tunnel Syndrome
Abstract and Introduction
Abstract
The American Academy of Orthopaedic Surgeons (AAOS) has developed Appropriate Use Criteria (AUC) for Management of Carpal Tunnel Syndrome. Evidence-based information, in conjunction with the clinical expertise of physicians, was used to develop the criteria to improve patient care and obtain best outcomes while considering the subtleties and distinctions necessary in making clinical decisions. To provide the evidence foundation for this AUC, the AAOS Evidence-Based Medicine Unit provided the writing panel and voting panel with the 2016 AAOS Clinical Practice Guideline titled Management of Carpal Tunnel Syndrome Evidence-Based Clinical Practice Guideline. The Management of Carpal Tunnel Syndrome AUC clinical patient scenarios were derived from indications typical of patients with suspected carpal tunnel syndrome in clinical practice, as well as from current evidence-based clinical practice guidelines and supporting literature to identify the appropriateness of treatments. The 135 patient scenarios and 6 treatments were developed by the writing panel, a group of clinicians who are specialists in this AUC topic. Next, a separate, multidisciplinary, voting panel (made up of specialists and nonspecialists) rated the appropriateness of treatment of each patient scenario using a 9-point scale to designate a treatment as Appropriate (median rating, 7 to 9), May Be Appropriate (median rating, 4 to 6), or Rarely Appropriate (median rating, 1 to 3).
Overview and Rationale
This Appropriate Use Criteria (AUC) was approved by the American Academy of Orthopaedic Surgeons (AAOS) Board of Directors on December 9, 2016.[1] The purpose of the AUC is to help determine the appropriateness of treatments of the heterogeneous patient population routinely seen in practice. The best available scientific evidence is synthesized with collective expert opinion on topics for which randomized clinical trials are not available or are inadequately detailed for identifying distinct patient types. AAOS staff convened two independent volunteer physician panels that developed this AUC.
The AAOS created this AUC as an educational tool to guide qualified clinicians through a series of diagnosis and management decisions in an effort to improve the quality and efficiency of care. These criteria should not be construed as including all indications or excluding indications reasonably directed to obtaining the same results. These criteria are intended to address the most common clinical scenarios facing all appropriately trained clinicians and were developed as guidelines, not to supersede clinician expertise, experience, or patient preference. The ultimate judgment regarding any specific criteria should address all circumstances presented by the patient and the needs and resources particular to the locality or institution. The AUC for the Management of Carpal Tunnel Syndrome developed appropriateness treatment ratings for 135 patient scenarios.
Potential Harms and Contraindications
The goal of the management of carpal tunnel syndrome is to aid in diagnosis and alleviate symptoms in affected patients. Many forms of management are associated with some potential for adverse outcomes, especially if invasive or surgical. Contraindications vary widely based on the treatment administered. Reducing risks improves treatment efficacy and is accomplished through collaboration and communication between patient and physician.
Methods
The AAOS uses the RAND/UCLA Appropriateness Method to develop the AUC.[2] Two panels participated in the development of the AAOS AUC for Management of Carpal Tunnel Syndrome. To provide the evidence foundation for this AUC, the AAOS Evidence-Based Medicine Unit provided the writing panel and voting panel with the 2016 AAOS Clinical Practice Guideline titled Management of Carpal Tunnel Syndrome Evidence-Based Clinical Practice Guideline, which can be accessed via the following link: www.orthoguidelines.org/ctsguideline.[3] Members of the writing panel developed a list of 135 patient scenarios, for which six treatments were evaluated for appropriateness. The voting panel participated in three rounds of voting. During the first round of voting, the voting panel was given approximately 2 months to independently rate the appropriateness of each of the provided treatments of each of the relevant patient scenarios via an electronic ballot. After the first round of appropriateness ratings was submitted, AAOS staff calculated the median ratings for each patient scenario. An in-person voting panel meeting was held in Rosemont, Illinois, on August 12, 2016. During this meeting, voting panel members addressed the scenarios/treatments that resulted in disagreement. The voting panel members discussed the list of assumptions, patient indications, and treatments to identify areas that needed to be clarified and/or edited. After the discussion and subsequent changes, the voting panel members were asked to re-rate their first-round ratings during the voting panel meeting, only if they were persuaded to do so by the discussion and available evidence. After completion of the second in-person round of voting, the voting panel opted to revisit the scenarios that still contained disagreement and reopen the electronic ballot for a third round of voting. The voting panel determined appropriateness by rating the appropriateness of treatment of the 135 patient scenarios as Appropriate, May Be Appropriate, or Rarely Appropriate. There was no attempt to obtain consensus about appropriateness.
This AUC was approved by the Committee on Evidence-Based Quality and Value, the Council on Research and Quality, and the AAOS Board of Directors. All tables, figures, and appendices, as well as the details of the methods used to prepare this AUC are detailed in the full AUC, which is available at http://www.aaos.org/ctsauc.[1]
Indications, Classifications, and Treatment
Table 1 provides the list of patient indications and classifications developed by the Management of Carpal Tunnel Syndrome AUC panels. The following treatment options are addressed within the AUC Management of Carpal Tunnel Syndrome: investigate alternative diagnosis; electrodiagnostic study (investigate further); nonsurgical treatment consisting of oral steroids or ketoprofen phonophoresis, nonsurgical treatment consisting of the use of a splint, nonsurgical treatment consisting of steroid injection; and carpal tunnel release (surgical treatment).
Table 1. Indications and Classifications| Indication | Classification |
|---|
| CTS diagnostic likelihood based on clinical examination | 1. Low Probability of CTS: CTS-6 Score of < 5(< 25% probability of CTS) and/or Unlikely CTS on Katz Hand Diagram
2. Moderate Probability of CTS: CTS-6 Score of 5–11.5 (25%–79% probability of CTS) and/or Probable/Possible CTS on Katz Hand Diagram
3. High Probability of CTS: CTS-6 Score ≥12 (80% probability of CTS) and/or Classic CTS on Katz Hand Diagram |
| Electrodiagnostic testing history | 1. No electrodiagnostic testing performed
2. Electrodiagnostic testing not consistent with carpal tunnel syndrome
3. Electrodiagnostic testing consistent with a mild median mononeuropathy at the wrist
4. Electrodiagnostic testing consistent with a moderate median mononeuropathy at the wrist
5. Electrodiagnostic testing consistent with severe median mononeuropathy at the wrist |
| Clinical severity | 1. Low Severity (examples: nighttime pain/sensory disturbances, and/or episodic/infrequent symptoms)
2. Moderate Severity (examples: pain/sensory disturbances, tingling, frequent activity-related symptoms, and/or difficulty with fine motor coordination)
3. High Severity (examples: constant sensory loss, motor clinical findings [eg, muscle weakness], and/or thenar atrophy) |
| Response to previous treatment | 1. No previous nonsurgical treatment of CTS
2. Positive response to nonsurgical treatment and subsequent recurrence of symptoms
3. Failure to respond to nonsurgical treatment |
CTS = carpal tunnel syndrome, CTS-6 = 6-item Carpal Tunnel Syndrome Evaluation Tool
Results of Appropriateness Ratings
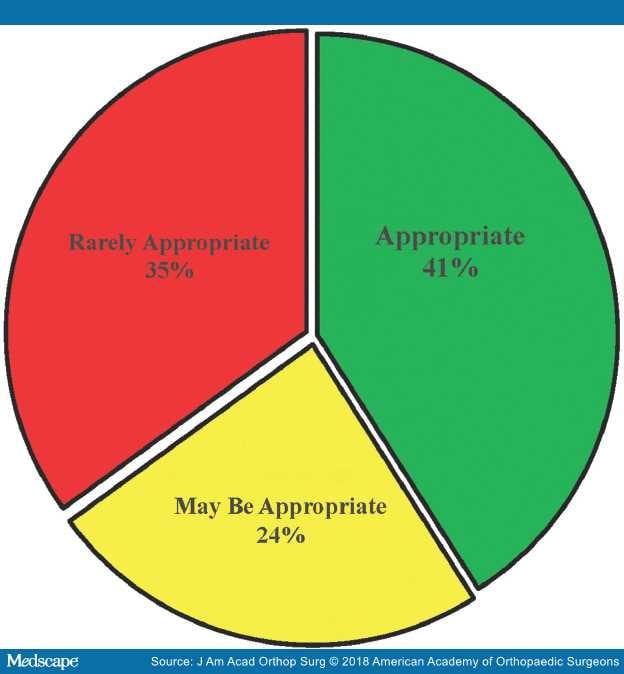
Of 810 total voting items (ie, 135 patient scenarios × 6 treatments), 284 voting items (35.06%) were rated as Rarely Appropriate, 196 voting items (24.2%) were rated as May Be Appropriate, and 330 voting items (40.74%) were rated as Appropriate (Figure 1). Additionally, the voting panel members were in agreement on 353 voting items (43.58%) and were in disagreement on 17 voting items (2.1%). The final appropriateness ratings assigned by the nine-member voting panel of the AAOS Management of Carpal Tunnel Syndrome AUC can be accessed via the web-based mobile application www.orthoguidelines.org.
Figure 1.
Summary of appropriateness ratings of the Management of Carpal Tunnel Syndrome Appropriate Use Criteria.
As part of the dissemination efforts for the Management of Carpal Tunnel Syndrome AUC, this web-based mobile platform was developed to provide physicians with immediate access to information to assist them with providing evidence-based patient care. The mobile platform includes the list of patient indications and treatment recommendations. After the clinician enters a patient indication profile specifying the carpal tunnel syndrome diagnostic likelihood based on clinical examination, electrodiagnostic testing history, clinical severity, and response to previous treatment, a list of treatment recommendations is provided. For the selected patient profile, green circled checkmarks reflect appropriate treatments, yellow caution symbols reflect treatments that may be appropriate, and red circled Xs reflect treatments that are rarely appropriate.
- American Academy of Orthopaedic Surgeons: Appropriate Use Criteria for the Management of Carpal Tunnel Syndrome. http://www.aaos.org/ctsauc. Published December 9, 2016. Accessed January 10, 2018.
- Fitch K, Bernstein SJ, Aguilar MD, et al: The RAND/UCLA Appropriateness Method User'sManual. Santa Monica, CA, RAND Corporation, 2001.
- American Academy of Orthopaedic Surgeons: Management of Carpal Tunnel Syndrome Evidence-Based Clinical Practice Guideline. https://www.aaos.org/ctsguideline. Published February 29, 2016. Accessed January 10, 2018.