B = treatment options are supported by fair evidence (consistent with level III or IV studies), C = treatment options are supported by conflicting or poor-quality evidence (level IV studies), I = existing evidence is insufficient to make a recommendation
Nonsurgical
Nonsurgical treatment options include NSAIDs, physical therapy, corticosteroid injections, and viscosupplementation.[27] These options should be attempted before surgical intervention because they are less invasive and may have beneficial effects in some patients. However, no studies have specifically attempted to assess the clinical outcomes of these nonsurgical interventions in patients with focal chondral defects of the glenohumeral joint. Thus, nonsurgical treatment warrants a grade I recommendation.
Surgical
For patients who continue to experience symptoms after an attempt at nonsurgical management of articular cartilage lesions in the shoulder, surgical treatment may be warranted. Several surgical procedures to manage these lesions have been described, including arthroscopic débridement, microfracture, osteochondral autograft transplantation, fresh osteochondral allograft transplantation, and autologous chondrocyte implantation. Implantation of particulated juvenile cartilage allograft, although yet to be reported on in the shoulder, has shown promising results in other joints[28,29] and may play a role in the management of chondral defects in the shoulder in the future.
Arthroscopic Débridement. Arthroscopic débridement consists of the removal of loose cartilage fragments during shoulder arthroscopy. In contrast to many of the other surgical techniques described in this review, arthroscopic débridement does not require the presence of full-thickness chondral lesions.
In a retrospective case series (level IV evidence), Kerr and McCarty[30]reviewed the results of 20 shoulders in 19 patients (mean age, 38 years) who underwent arthroscopic débridement of glenohumeral cartilage lesions with an Outerbridge grade of 2 to 4. At a mean follow-up of 20 months, three patients required shoulder arthroplasty, and therefore their treatment was considered unsuccessful. For the remaining patients, the mean Single Assessment Numeric Evaluation score, American Shoulder and Elbow Surgeons (ASES) score, and Western Ontario Osteoarthritis Score were 71%, 75.3, and 0.64, respectively, at follow-up. Patients with unipolar lesions had a significantly higher mean Western Ontario Osteoarthritis Score, ASES score, and Single Assessment Numeric Evaluation score (P = 0.014, P = 0.033, and P = 0.022, respectively) at follow-up, compared with patients with bipolar lesions. The grade of lesion did not affect outcome scores.
In another retrospective case series (level IV evidence), Skelley et al[31]evaluated 33 patients (mean age, 55 years) who had undergone arthroscopic débridement and capsular release for the management of shoulder osteoarthritis (Outerbridge grade 2 to 4 cartilage lesions). Fourteen patients (42%) required total shoulder arthroplasty at a mean of 8.8 months after arthroscopy. Among the remaining patients, mean ASES scores improved from 42.2 preoperatively to 50.8 at final follow-up, although this finding was not statistically significant (P = 0.41). Similarly, mean visual analog scale (VAS) pain scores improved from 7.8 to 7.4; this finding was not significant (P = 0.59).
Van Thiel et al[32] reported on a retrospective case series (level IV evidence) of 81 patients who underwent arthroscopic débridement for the management of glenohumeral arthritis, of whom 71 patients (mean age, 47 years) were available for follow-up. At follow-up, 16 patients had undergone shoulder arthroplasty at a mean of 10.1 months postoperatively. No significant differences were found between the arthroplasty and nonarthroplasty cohorts with respect to sex or age. However, a reduced preoperative joint space in the arthroplasty group was evident on plain radiographs (1.5 mm versus 2.6 mm; P < 0.05). Among the nonarthroplasty patients, substantial improvements from preoperative scores were demonstrated in the ASES score, Simple Shoulder Test (SST) score, and VAS pain score at a mean follow-up of 27 months.
Because of the fair results of three level IV studies, the use of arthroscopic débridement for the removal of loose cartilage fragments in the glenohumeral joint warrants a grade C recommendation.
Microfracture. Microfracture in the glenohumeral joint is often performed using the same technique used in other joints, such as the knee. As with débridement, this procedure can be performed arthroscopically. After débridement of loose cartilage flaps and the calcified cartilage layer to confirm the presence of a contained lesion, a microfracture awl is used to penetrate the subchondral bone perpendicular to the bone surface, starting at the periphery of the lesion and proceeding toward the center (Figure 1). Typically, each microfracture hole is spaced 3 to 4 mm apart and penetrated to a depth of 2 to 4 mm.[33] Adequate spacing between the holes is critical to avoid fracture between penetrations. Finally, the irrigation pump pressure is reduced to allow the bone marrow elements (containing growth factors and stem cells) to emerge from the subchondral perforations.
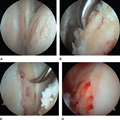
Figure 1.
Arthroscopic images demonstrating surgical management of a glenohumeral bipolar chondral defect. Management of the chondral defect (A) included microfracture of the humeral head defect (B) and microfracture of the glenoid defect (C). D, The appearance of the humeral head after microfracture with release of marrow elements. (Courtesy of Eric McCarty, MD, Aurora, CO.)
In one case series (level IV evidence), Frank et al[33] described the clinical outcomes of 17 shoulders in 16 patients (mean age, 37.0 years) who underwent arthroscopic microfracture of the humeral head and/or glenoid. Average defect sizes were 5.1 cm2 for the humeral head and 1.7 cm2 for the glenoid. At a mean follow-up of 27.8 months, two patients were lost to follow-up. Among the remaining patients, the mean VAS decreased significantly, from 5.6 preoperatively to 1.9 at follow-up (P < 0.01). The mean SST score improved significantly, from 5.7 preoperatively to 10.3 at follow-up, and the mean ASES score improved from 44.3 to 86.3 (both P < 0.01). Three patients required subsequent shoulder surgery, and therefore the microfracture treatment was considered unsuccessful.
In another case series (level IV evidence), Millett et al[34] performed microfracture in 31 shoulders in 30 patients (mean age, 43 years). Microfracture was performed on only the humeral head in 12 shoulders, on only the glenoid in 13 shoulders, and on both surfaces in 6 shoulders. Six of the 31 shoulders (19%) required additional surgery, including three total shoulder arthroplasties. At a mean follow-up of 47 months, the mean pain score had decreased from 3.8 preoperatively to 1.6, and the mean ASES score had improved from 60 to 80 (both P < 0.001). Patients with isolated microfracture of the humerus demonstrated the greatest improvements.
Snow and Funk[35] reported on a case series (level IV evidence) in which they performed arthroscopic microfracture in eight patients (mean age, 37 years) with full-thickness chondral defects of the glenohumeral joint. Five patients had humeral head defects, and three patients had glenoid defects. The mean Constant score improved from 44 preoperatively to 90 at a mean follow-up of 15 months (P < 0.005). No complications were reported. Two patients underwent subsequent shoulder surgery, one for revision SLAP repair and one for subacromial decompression. In both patients, good cartilage filling was seen at the site of microfracture.
Because of the positive results demonstrated in several case series, the use of arthroscopic microfracture in the management of glenohumeral chondral defects warrants a grade B recommendation.
Osteochondral Autograft Transplantation. Osteochondral autograft transplantation involves harvesting one to three osteochondral autograft plugs, typically from a non–weight-bearing portion of the knee joint, and using a press-fit technique to transfer these plugs into the chondral defect site.[36] An advantage of this technique is that the resulting cartilage repair is native hyaline cartilage, although no healing occurs at the border of the osteochondral plugs between the autograft and the host cartilage.[37]Whereas simple débridement and microfracture can be performed arthroscopically, osteochondral autograft transplantation requires an open approach to gain full access to the joint to transfer the osteochondral plugs.
In a case series (level IV evidence), Scheibel et al[36] described the clinical results of eight patients (mean age, 43 years) who underwent osteochondral autograft transplantation for the management of full-thickness cartilage defects of the shoulder (mean size, 1.5 cm2). Seven patients had a humeral-sided defect. Osteochondral plugs were harvested from the outer edge of the lateral femoral condyle. At a mean follow-up of 32.6 months, significant improvement was demonstrated in the Constant score, from a mean score of 73.9 preoperatively to 88.7 at follow-up (P < 0.05). In seven of the eight patients, MRI demonstrated excellent graft viability and congruence of the chondral surfaces. Second-look arthroscopy was performed in two patients to assess the chondral surfaces. Both patients had good macroscopic integration of the grafts, with the original chondral defects completely covered by cartilage tissue.
Among the same group of patients studied by Scheibel et al,[36] Kircher et al[37] described the clinical results of seven of the eight patients at a mean follow-up of 8.8 years. One patient was lost to follow-up after complaints involving the donor site in the ipsilateral knee. None of the remaining seven patients required revision surgery of the shoulder or the donor knee. The mean Constant score improved from 76.2 preoperatively to 89.6 at a mean 32.6-month follow-up and to 90.9 at final follow-up. The Lysholm score was used to assess the effects of the harvesting of the osteochondral plug from the ipsilateral knee. A modest decline in the average score, from 100 points preoperatively to 99.3 at final follow-up, was found. In all but one patient, MRI demonstrated a congruent joint surface. A significant increase in the osteoarthritic grade of the shoulder according to Samilson and Prieto[38] was demonstrated from preoperatively to the first follow-up (P = 0.014) but not between the first and final follow-up times (P = 0.157). All patients showed an increase of at least one osteoarthritic grade from surgery to the first follow-up.
Because of the overall beneficial effects demonstrated at long-term follow-up in these two studies, the use of osteochondral autograft transplantation for the management of focal chondral defects in the glenohumeral joint warrants a grade C recommendation.
Fresh Osteochondral Allograft Transplantation. Fresh osteochondral allograft transplantation uses the same concepts as osteochondral autograft transplantation. The advantages of allografts are a shorter surgical time and decreased morbidity associated with harvesting an autograft osteochondral plug. Thus, fresh osteochondral allograft transplantation may be beneficial for the management of larger lesions that cannot be adequately managed with osteochondral autograft plugs. The procedure requires a suitable cadaver donor with similar articular geometry for a matched fit. Although glenoid allografts are not currently available, a recent study demonstrated that the articular geometry of the medial tibial plateau closely resembles that of the glenoid.[39] As with osteochondral autograft transplantation, osteochondral allograft transplantation typically is performed as an open procedure using a deltopectoral approach.[40] However, all-arthroscopic techniques for osteochondral allograft transplantation with the use of tibial plateau allograft and an Osteochondral Autograft Transfer System (Arthrex) have been described.[41]
Recently, Riff et al[40] reported on a series of 20 patients who underwent osteochondral allograft transplantation for the management of humeral head osteochondral defects. Humeral head allografts were used for all patients. The average age at the time of surgery was 24.8 years (SD, 8.1 years). Eleven of the 20 patients (55%) underwent concomitant microfracture or meniscal allograft resurfacing of the glenoid. Eighteen patients (90%) were available for follow-up at a minimum of 24 months (mean, 67 months). Four patients underwent shoulder arthroplasty at a mean of 25 months postoperatively. The authors reported significant improvements in several outcome scores at final follow-up: VAS (6.1 preoperatively to 1.5 postoperatively), SST (32 preoperatively to 73 postoperatively), ASES (39 preoperatively to 76 postoperatively), and Medical Outcomes Study 12-Item Short Form physical component summary (38 preoperatively to 48 postoperatively). Patients with a history of using an intra-articular pain pump experienced significantly inferior patient satisfaction compared with patients who had no history of pain pump use (40% versus 87.5%; P = 0.04).
Diklic et al[42] reported on a series of 13 patients (mean age, 42 years) with chronic posterior shoulder dislocation and an associated humeral head osteochondral defect of 25% to 50% of the articular surface. All patients underwent osteochondral allograft transplantation with a femoral head allograft. At a mean follow-up of 54 months (range, 41 to 64 months), nine patients (69%) had no pain or restriction of activities of daily living. Osteonecrosis of the head of the humerus developed in one patient, who required oral analgesic agents for pain relief. Three additional patients reported occasional, mild night pain without the need for analgesia.
In addition to humeral head and femoral head allografts, the use of iliac crest bone allograft has been described.[43] DiPaola et al[43] reported on five patients with anterior shoulder instability who underwent osteochondral allograft transplantation of the humeral head. Four of these patients were available for follow-up at a mean of 28 months postoperatively (range, 11 to 40 months). At follow-up, the average ASES and UCLA scores were 85.3 and 28.4, respectively. Compared with the contralateral, normal shoulders, the operated shoulders lost an average of 23° of forward flexion, 8° of external rotation, and two vertebral levels of internal rotation.
Several case reports have described the use of osteochondral allograft transplantation for the management of glenohumeral articular cartilage lesions, with beneficial clinical outcomes demonstrated in all cases.[20,22,39,44–46] The use of osteochondral allograft transplantation has been described for the management of glenoid defects, humeral head defects, and bipolar focal chondral defects. Because of the beneficial outcomes reported in several small case series, fresh osteochondral allograft transplantation warrants a grade C recommendation.
Autologous Chondrocyte Implantation. Autologous chondrocyte implantation involves a two-stage technique. During the first procedure, a small biopsy sample of healthy cartilage is obtained, either from the border of the chondral defect[47] or from the knee joint.[21] The chondrocytes in this biopsy sample are cultured in vitro over several weeks. During the second procedure, an arthrotomy is necessary to gain access to the defect. The arthrotomy is typically performed through a deltopectoral approach with takedown of at least a portion of the subscapularis tendon, depending on the location of the defect. Depending on the autologous chondrocyte implantation technique, the chondrocytes grown from the biopsy sample are either injected under a periosteal patch (first-generation autologous chondrocyte implantation) or seeded onto a collagen membrane cut to the shape and size of the defect (third-generation autologous chondrocyte implantation). In the latter method, the seeded layer of the membrane is placed on the subchondral bone within the defect and is fixed with absorbable sutures and/or fibrin glue.
In a case series (level IV evidence) of four consecutive male patients (mean age, 29 years), Buchmann et al[47] performed autologous chondrocyte implantation using a type I/III collagen-based membrane. Three of the four patients had a humeral-sided full-thickness cartilage defect (each measuring 6.0 cm2), and one patient had a glenoid full-thickness defect of 2.0 cm2. At a mean 41-month follow-up, the mean VAS pain, Constant, and ASES scores were 0.3 of 10, 83.3, and 95.3, respectively. Preoperative scores were not available for comparison. The magnetic resonance observation of cartilage repair tissue score was also used and indicated overall satisfactory defect coverage with signs of fibrocartilaginous repair tissue.
Romeo et al[21] described the use of first-generation autologous chondrocyte implantation for the treatment of a 16-year-old boy with a 3.3-cm × 1.5-cm full-thickness cartilage defect on the anterosuperior aspect of the humeral articular surface. A periosteal patch was harvested from the medial tibia and sewn to the healthy cartilage border surrounding the defect with the use of absorbable braided 6–0 sutures, with an opening left to allow injection of the autologous chondrocytes. Fibrin glue was applied to the periphery of the periosteal patch to seal the surface. The patient was allowed progressive range of motion for 12 weeks postoperatively. At 12-month follow-up, the patient demonstrated full, painless range of motion with no further complaints. However, no standardized outcome scores were reported. Because of the paucity of evidence apart from these small case reports, autologous chondrocyte implantation warrants a grade I recommendation.
Particulated Juvenile Allograft Cartilage. Recent studies have reported promising results with the use of particulated juvenile allograft cartilage to manage chondral defects in the ankle, knee, and hip.[28,29,48] The indications for this technique are similar to those of autologous chondrocyte implantation. However, particulated allograft cartilage implantation has the advantage of being a single-stage procedure. Particulated fragments and fibrin glue are used to make a mold to fit the cartilage defect. The mold is implanted and secured with fibrin glue with or without a collagen I/III membrane. No reports of the use of this technique for the management of glenohumeral chondral defects are available. Thus, particulated allograft cartilage implantation is given a grade I recommendation.
Summary
Although articular cartilage lesions are encountered less frequently in the glenohumeral joint than in other joints, management of these lesions remains a challenging clinical problem. The literature lacks high-quality evidence regarding the nonsurgical and surgical treatment options for patients with these chondral defects. Additional high-quality comparative studies are necessary to determine which treatment strategies provide the best long-term outcomes for patients with glenohumeral articular cartilage lesions.