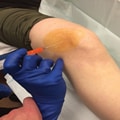
Photograph showing the ultrasonography-guided suprapatellar approach to the knee joint at the superolateral border of the patella.
Proximal Tibiofibular Joint
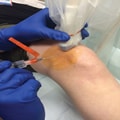
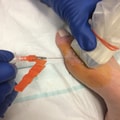
The proximal tibiofibular joint (PTFJ) consists of the articulation between the medial aspect of the fibular head and the proximal posterolateral tibia. It is a less common and potentially overlooked etiology of lateral knee pain. Pathology at this location may be due to arthritis, injury, compression of the common peroneal nerve, or a symptomatic ganglion cyst.[21,22] Injection of the joint may be conducted for diagnostic and therapeutic purposes and performed by palpation alone or with image guidance. Positioning the patient in the lateral decubitus position with the knee slightly flexed may facilitate needle placement (Table 2; Figure 3). Smith et al[23] reported a comparison of palpation-guided injections and US-guided techniques for the PTFJ in a cadaver model. The authors reported 100% accuracy with image guidance versus 58% with a palpation-guided technique. Inaccurate placement was superficial and inferior to the PTFJ in all cases of unsuccessful injection, with extravasation into the adjacent musculature. Only two palpation-guided injections delivered all the fluid into the PTFJ (17%).[23]
Photograph showing the ultrasonography-guided approach to aspiration or injection of the proximal tibiofibular joint.
Pes Anserine Bursa
The pes anserinus is a confluence of the sartorius, gracilis, and semitendinosus tendons onto the proximal anteromedial tibia. A potential bursa lies between the pes anserinus tendons and the more deeply located medial collateral ligament and/or medial tibia. Pain in the area of the pes anserine bursa is most commonly secondary to an inflammation of the bursa, tenosynovitis, or tendinopathy as a result of repetitive overuse or direct trauma. Injection may be considered as a diagnostic tool or as a treatment modality for recalcitrant pain.
The confluence and its bursa are best palpated on the anteromedial aspect of the proximal tibia. Alternatively, the tendons and bursa may be visualized using US. Patients are placed in the lateral decubitus position, with the knee slightly flexed to facilitate needle placement (Table 2). Despite the superficial location of the pes anserinus, unguided bursa injections have proved less accurate than US-guided injections. In a single-blinded, prospective study, Finnoff et al[8] reported a markedly different accuracy of 92% versus 17%, respectively, when comparing the US-guided versus palpation-guided technique in adult cadaver specimens.
Foot and Ankle Region
Tibiotalar Joint
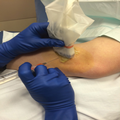
The tibiotalar joint is a common site of arthritis, synovitis, osteochondral injury, and impingement. Both the anterolateral and anteromedial approaches to the tibiotalar joint are useful to access the joint (Table 2; Figure 4). The needle is placed just medial to the tibialis anterior tendon for the anteromedial approach and just lateral to the peroneus tertius for the lateral approach.
Photograph showing the palpation-guided anteromedial approach to aspiration or injection of the ankle joint.
With regard to the use of image guidance, Wisniewski et al[24] found superior accuracy of US-guided versus nonguided anteromedial tibiotalar joint injections (100% versus 85%, respectively) in a cadaver model. Reach et al[25] similarly reported 100% accuracy of US-guided anteromedial injections to the tibiotalar joint; however, no comparison group existed.
Subtalar Joint
The subtalar (talocalcaneal) joint allows for inversion and eversion of the hindfoot. Three anatomic approaches have been described (ie, anterolateral, posterolateral, posteromedial), with the posterolateral often preferred because of its distance from neurovascular structures[5,9] (Table 2). Reach et al[25] reported slightly less accuracy (90%) in their investigation of US-guided injection of the subtalar joint in the cadaver model. Henning et al[9]evaluated the accuracy of three US-guided approaches (ie, anterolateral, posteromedial, posterolateral) to inject the posterior subtalar joint and found that all three approaches provided accurate needle placement while also minimizing the risk of needle entry into adjacent soft-tissue structures. In a comparison of the palpation-guided anterolateral and the posterolateral approach in 68 cadaver models, 23 (67.7%) of the anterolateral injections were successful compared with 31 (91.2%) of the posterolateral injections.[26]The greater accuracy of the posterolateral approach was statistically significant (P = 0.016).
Peroneal Tendon Sheath
In the supramalleolar region, the peroneal tendon complex comprises the peroneus longus tendon and the more medial peroneus brevis muscle and tendon. As the complex courses distally, the peroneus longus courses posterior to the brevis tendon. Trauma to the tendons occurs from a forceful contraction of the muscles, with the foot in plantar flexion and inversion. Anatomic variations, such as a shallow or flat retrofibular groove, which houses the tendons as they course behind the fibula, may contribute to persistent or recurrent subluxation of the tendons. Tenosynovitis or tendinopathy occurs secondary to trauma or repetitive microtrauma. Anesthetic injections may be used for diagnostic purposes to assist in the clinical decision-making process and to assess for surgical appropriateness.
The patient is positioned supine, with the hip internally rotated and a towel roll placed under the medial aspect of the ankle (Table 2). Injections to the sheath are performed via palpation or with image-guided assistance. In one cadaver study, US-guided peroneal tendon sheath injections were markedly more accurate than palpation-guided injections (100% versus 60%, respectively).[27] Accuracy is particularly important in the peritendinous injection to minimize the chance of an intratendinous injection, particularly if corticosteroid is used. Reach et al[25] similarly reported 100% accuracy with US guidance for injections of the posterior tibialis and flexor hallucis longus tendon sheaths.
Midfoot and Forefoot
Khosla et al[28] reported on the accuracy of intra-articular injections using palpation versus dynamic US in a cadaver model and reported 100% accuracy in subtalar and ankle joint injections in both techniques. However, using palpation, the needle was correctly placed into the first transmetatarsal joint in 3 of 14 cadavers, compared with 10 of 14 cadavers using US. Similar results were obtained with placement into the second transmetatarsal joint (ie, four with palpation versus eight with US).
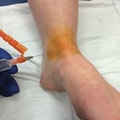
The first metatarsophalangeal (MTP) joint comprises the articulation between the first metatarsal and the proximal phalanx of the hallux. Pain at the MTP joint may be acute or chronic in nature and the result of trauma, gout, or other inflammatory arthritides. Variation exists in the size and shape of MTP joints; therefore, palpation in the setting of conditions such as advanced degenerative arthritis may prove challenging.[29] If necessary, distraction of the joint is helpful during needle placement (Table 2). Diagnostic aspiration or therapeutic injection may be useful in the management of advanced osteoarthritis, rheumatoid arthritis, and gout (Figure 5).
Photograph showing the ultrasonography-guided approach to aspiration or injection of the first metatarsophalangeal joint.
Few data are available on the comparison between palpation and image-guided techniques for injection or aspiration of the first MTP joint. Reach et al[25] report 100% accuracy when using US guidance; however, this analysis was performed without a comparison group. Balint et al[10] reported markedly lower accuracy rates in the conventional, palpation-guided technique for joint and soft-tissue aspiration compared with the US-guided technique. In the conventional group, successful aspiration was achieved in only 32% of the joints, compared with 97% of the aspirations in the US-guided group. The mean volume of fluid obtained with successful aspirations was similar in both groups.
Synovial Fluid Analysis
Joint fluid analysis is a useful diagnostic tool in the management of both septic arthritis and inflammatory disease. Analysis of the aspirate is critical in the treatment of adult septic arthritis because it guides antibiotic management. It also allows for the establishment of an accurate diagnosis in crystalline disease and guides management of inflammatory arthritis. The macroscopic appearance of synovial fluid provides immediate information to apply toward the differential diagnosis. The color, clarity, and viscosity can be appreciated on gross examination (Table 3). Synovial fluid specimens are placed in specimen containers specific to the test being ordered. A heparinized tube is preferable for cell counts, sterile containers for microbiology testing, and plain tubes for chemistry and immunological testing of the fluid. The volume aspirated from the joint may be small, and if only a few milliliters of fluid are available, preference should be given for cell count analysis. Normal synovial fluid is straw-colored, clear, and viscous. Increased inflammation changes the fluid's macroscopic appearance; the color ranges from yellow to greenish yellow, the clarity is more opalescent, and the viscosity is decreased. In the setting of a pyogenic infection, the aspirate may appear as frank, purulent material. Bloody fluid may be the result of joint trauma, coexisting anticoagulation therapy, baseline coagulation disorders, or synovial tumors such as pigmented villonodular synovitis.[3] The appearance of particles other than cells may indicate the presence of crystals. In rare instances, concomitant septic and gouty arthritis exists;[2] therefore, early diagnosis requires a high level of suspicion because there may be an absence of fever or leukocytosis. Synovial fluid aspirate should be sent for cell count and differential, Gram stain, microbiologic culture, and crystal analysis,[1] with results guiding subsequent management (Table 3). Of note, the cell count suggestive of septic arthritis in a prosthetic joint is much lower compared with a native joint. Additional biochemical studies, including the analysis of glucose, protein, and complement level, may be performed to provide further characterization of inflammatory arthritides.
Summary
Aspiration and injection of joints and soft tissues are safe and effective techniques for the diagnosis and treatment of musculoskeletal disorders. Intra-articular aspiration provides invaluable diagnostic information in cases of septic arthritis and inflammatory arthritides. Injection of local anesthetic may be of great utility for diagnostic purposes, and corticosteroid injections may be both diagnostic and therapeutic. Although correct needle placement in most of the peripheral joints of the lower extremity is routine in the hands of the experienced clinician, the use of image guidance to localize joint and soft-tissue structures may be beneficial. Correct needle placement has important implications for the administration of local treatment and underscores the significance of US as a useful device in clinical practice.