Rachel M. Frank, MD; Eric J. Cotter, BS; Eric J. Strauss, MD; Andreas H. Gomoll, MD; Brian J. Cole, MD, MBA
J Am Acad Orthop Surg. 2018;26(1):e11-e25.
The management of complex cartilage and meniscal pathology in young, athletic patients is extremely challenging. Joint preservation surgery is most difficult in patients with concomitant knee pathologies, including cartilage defects, meniscal deficiency, malalignment, and/or ligamentous insufficiency. Clinical decision making for these patients is further complicated by articular cartilage lesions, which often are incidental findings; therefore, treatment decisions must be based on the confirmed contribution of articular cartilage lesions to symptomatology. Surgical management of any of the aforementioned knee pathologies that is performed in isolation typically results in acceptable patient outcomes; however, concomitant procedures for the management of concomitant knee pathologies often are essential to the success of any single procedure. The use of biologic therapy as an alternative to or to augment more conventional surgical management has increased in popularity in the past decade, and indications for biologic therapy continue to evolve. Orthopaedic surgeons should understand knee joint preservation techniques, including biologic and reconstructive approaches in young, high-demand patients.
The management of complex knee pathology in young, athletic patients is challenging. Various joint preservation strategies have been introduced in the past several decades, with biologic therapy recently being incorporated into the treatment algorithm for complex knee pathology. Although successful outcomes can be achieved in patients with complex knee pathology who undergo nonsurgical treatment, most patients require surgical treatment to preserve and/or restore joint biomechanics and function. The ability to perform complex and concomitant knee joint preservation procedures in these patients is increasing given recent advances in surgical techniques, instrumentation, and imaging modalities, as well as the availability of off-the-shelf implants and biologic agents.
One of the main challenges in the treatment of patients with multiple knee pathologies is determining which pathology is symptomatic, which pathology must be managed (even if asymptomatic), and which pathology can remain unmanaged. Although every effort should be made for joint preservation in these patients, disadvantages, including inherent surgical risks and unique rehabilitation protocols, are associated with each joint preservation technique; therefore, care must be taken to avoid overmanagement of asymptomatic lesions. Surgical decision making is challenging in patients with tibiofemoral malalignment, ligamentous instability, and chondral/meniscal damage; therefore, all joint preservation options must be considered.[1] Historically, corrective procedures for the management of any of the aforementioned knee pathologies that are performed in isolation result in adequate patient outcomes; however, concomitant procedures for the management of concomitant knee pathologies often are essential to the success of any single procedure. Some patients may have limited access to timely care, especially with respect to allograft availability, and orthopaedic surgeons must account for potential disparities in healthcare access with regard to surgical decision making.
The last option for patients with debilitating and advanced joint line pain is joint arthroplasty, such as unicompartmental knee arthroplasty or total knee arthroplasty (TKA), both of which result in consistent pain relief and restoration of function in appropriately selected patients. Although knee arthroplasty is effective, such procedures are not ideal for younger and/or active patients, especially those with moderate- to high-demand activity levels.[1] Younger age has been reported to be a negative prognostic factor for clinical outcomes and revision surgery in patients who undergo knee arthroplasty,[2–4] which highlights the importance of knee joint preservation rather than knee replacement in these patients. Orthopaedic surgeons must determine the chronologic and physiologic age of patients in whom joint preservation procedures are considered. For example, older patients who historically may have been considered candidates for joint arthroplasty only may be excellent candidates for joint preservation surgery, depending on their weight, overall health, activity level, and surrounding joint anatomy. Conversely, younger patients who historically would never be considered candidates for joint arthroplasty because they are too young may not be good candidates for joint preservation based on their weight, overall health, postoperative expectations, and overall joint health. An understanding of the potential activity restrictions after joint preservation procedures is particularly important for younger patients, who are more likely than older patients to place higher demands on their joints postoperatively.
Patients with complex knee pathology often have one or more of the following diagnoses: meniscal insufficiency, articular cartilage lesions, ligamentous instability, and/or malalignment. These concomitant pathologies often are linked, with one underlying pathology being a strong contributor to a successive pathology, such as meniscal insufficiency leading to cartilage damage. In general, patients with a history of knee injury have a 7.4 times increased risk of knee osteoarthritis progression compared with patients without a history of knee injury.[5]
Injury to the meniscus is a particular problem for the future health of a knee. Baratz et al[6] reported that patients who underwent total meniscectomy experienced increased peak contact stresses of >235% compared with patients who had knees with intact menisci. Lee et al[7] reported a linear relationship between increases in knee contract stresses and the extent of partial meniscectomy. Many studies have described the detrimental effect of meniscectomy with regard to knee arthritis progression, reporting that patients who undergo total or subtotal meniscectomy have a 14 times increased relative risk of unicompartmental arthritis.[8–10] Inferior outcomes in patients who undergo partial meniscectomy have been associated with younger age, chondral damage discovered at the time of meniscectomy, ligamentous instability, and tibiofemoral malalignment.[11–13] In addition, the results of meniscal repair and meniscal allograft transplantation (MAT) are poorer in patients with unmanaged ligamentous instability, malalignment, and/or articular cartilage disease.[1,14–17] Concurrent knee pathologies have a considerable effect on the results of meniscal procedures.
Although articular cartilage damage may result from meniscal insufficiency, articular cartilage lesions also occur in isolation. Overall, articular cartilage injuries are extremely common, with studies reporting cartilage lesions in 60% to 65% of patients who undergo knee arthroscopy regardless of the surgical indication.[18–20] The effects of full-thickness articular cartilage defects on a knee may be considerable. These lesions have been reported to alter the distribution of weight-bearing forces in the knee; concentrate stresses at the rim of the defect and on the opposing articular surface; decrease overall contact area, which further increases peak stresses; and lead to degenerative changes and symptoms in the knee.
Ligamentous instability, particularly with regard to the anterior cruciate ligament (ACL), may contribute to degenerative changes in the knee.[21] In a recent study of 364 patients with an isolated ACL tear who underwent nonsurgical treatment, Sanders et al[22] reported hazard ratios of 18 and 14.2 with regard to the risk of secondary meniscal tears and arthritis, respectively, compared with an age- and sex-matched cohort of persons without an ACL tear. Lohmander et al[23] reported that 50% of patients with ACL and meniscal tears had symptomatic osteoarthritis 10 to 20 years postinjury. In a study of 56 patients with an ACL tear (with or without a concomitant meniscal tear), Zhang et al[24] reported considerably less flexion during gait analysis in all of the ACL-deficient knees, regardless of the status of the meniscus, compared with ACL-intact knees. In addition, the authors reported that concomitant meniscal injury altered knee kinematics by allowing for an increase in anterior tibial translation during level walking activity, which further highlights the link between multiple knee pathologies.[24]
Malalignment is a common source of knee pain and may have detrimental effects on the overall health of a knee. Under normal physiologic conditions, 60% of body weight is supported by the medial compartment of the knee, and 40% of body weight is supported by the lateral compartment of the knee. Varus malalignment results in overload of the medial compartment of the knee, whereas valgus malalignment results in overload of the lateral compartment of the knee. Varus malalignment has been reported to predict a loss of medial tibial plateau cartilage volume and an increase in tibial and femoral denuded bone.[25] Malalignment may be clinically asymptomatic, becoming clinically relevant only after a patient sustains an injury that results in pain and/or mechanical symptoms localized to one of the knee compartments. A common clinical scenario involves a previously asymptomatic patient who has lived his or her entire life with varus malalignment and sustains an injury that results in a new, large, symptomatic medial femoral condyle chondral defect. After the decision is made to proceed with surgical management of the chondral defect, surgeons must determine if a concomitant high tibial osteotomy (HTO) should be performed to correct the varus malalignment and offload the newly managed medial femoral condyle lesion (Figure 1). The same surgical decision making must be applied to the management of newly symptomatic lesions in the lateral compartment of the knee of a patient with valgus malalignment.
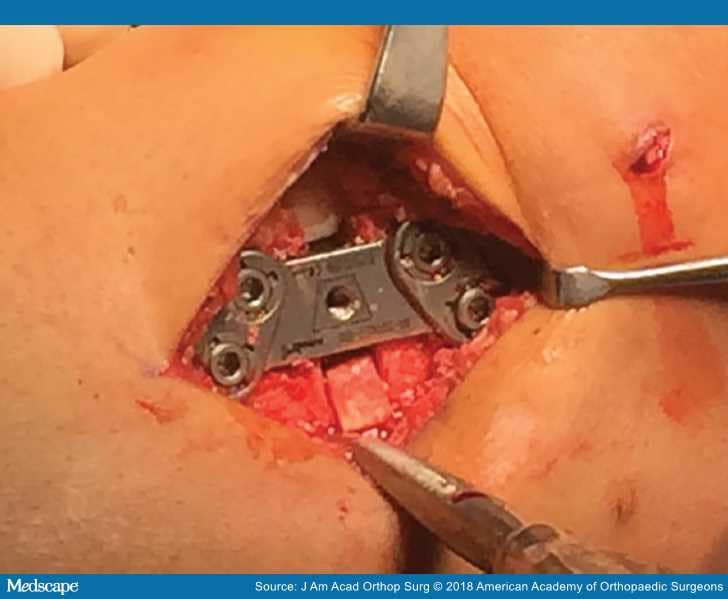
Figure 1.
Intraoperative photograph of a knee with varus malalignment showing plate fixation during a high tibial osteotomy.
The benchmark for the management of malalignment, whether performed in isolation or via a combined approach, is a realignment osteotomy, with an HTO most commonly performed for the management of varus malalignment and a distal femoral osteotomy most commonly performed for the management of valgus malalignment. Although an osteotomy often is necessary to allow for successful concomitant joint preservation strategies, osteotomy is associated with risks, including infection, fracture, nonunion, malunion, hardware failure, and neurovascular damage.[26,27] Although TKA is more technically challenging in patients in whom an osteotomy was performed, the clinical outcomes and survival rates of patients who undergo TKA after an osteotomy are similar to those of patients who undergo TKA without a prior osteotomy.[28–31]
The evaluation of patients with concomitant knee pathologies has been described in detail.[32–34] In general, evaluation of this patient population may be difficult, even for experienced surgeons. Patients with concomitant knee pathologies often have undergone multiple prior ipsilateral knee surgeries, and it may be difficult to determine whether a patient's current symptoms are related to an original injury, prior surgery, or a new injury. In addition, in patients with multiple known pathologies, such as articular cartilage and meniscal damage, it may be difficult to determine the lesion that contributes to most of a patient's symptoms. Common history and physical examination findings in patients with concomitant knee pathologies are listed in Table 1.
Diagnostic studies, including radiographs and advanced imaging studies, are extremely helpful in the evaluation of patients with complex knee pathology (Table 2). The initial diagnostic workup should always include weight-bearing, double-stance, long-leg mechanical axis radiographs to evaluate the alignment of the limb in question (Figure 2). If available, surgical reports and intraoperative images and/or video from prior arthroscopic procedures should be reviewed to better understand procedures that already have been attempted and may provide important details with regard to the reason prior procedures have been unsuccessful (Figure 3). The details obtained from the patient history, physical examination, and imaging studies should help orthopaedic surgeons determine if a patient is an appropriate candidate for knee joint preservation surgery. Understanding patient expectations and counseling patients and families with regard to potential activity restrictions after knee joint preservation surgery are important to ensure successful outcomes that are satisfactory to patients.
AP weight-bearing, double-stance, long-leg mechanical axis radiograph of a lower extremity demonstrating mild varus malalignment.
Intraoperative arthroscopic image of a large medial femoral condyle fullthickness articular cartilage defect (International Cartilage Repair Society grade IV).
In general, patients in whom knee joint preservation techniques are considered often have debilitating pain and limited function. Usually, these patients have undergone one or more prior ipsilateral surgical procedures and are seeking a surgical alternative to nonsurgical treatment. Nonsurgical management options that may help alleviate pain include weight loss, activity modification, oral anti-inflammatory medications, unloader bracing, cryotherapy, compression therapy, physical therapy, injection therapy via intra-articular corticosteroids, viscosupplementation, and biologic injections. Although nonsurgical treatment can be attempted if a patient prefers, most patients have severe symptoms and pathology that warrant surgical treatment. Surgical management options for knee joint preservation vary and depend on the specific pathology or pathologies being managed. A summary of the surgical techniques for knee joint preservation, which can be performed in isolation or via a combined approach, is presented in Table 3.
Depending on patient indications, surgical techniques for knee joint preservation can be performed concurrently or in a staged manner (Figure 4). A concurrent approach requires a single surgery but often requires a longer surgical duration, which may be associated with a more difficult postoperative recovery. A staged approach is advantageous because it involves a shorter surgical duration and a potentially easier postoperative recovery but requires multiple surgeries, each of which is associated with anesthetic and surgical risks. The cost-effectiveness of concurrent versus staged knee joint preservation procedures has not been evaluated in enough detail to determine the superiority of one approach over the other. Although determining the most appropriate procedures for the management of meniscal deficiency, ligamentous instability, and malalignment is somewhat straightforward, determining the optimal procedure for the management of a full-thickness chondral lesion is more controversial.[33,35–37] The continuous development of novel, biologic solutions further complicates the decision-making process because such treatments often offer attractive, less-invasive options compared with traditional techniques but lack clinical evidence to support their use. Overall, it is extremely difficult to determine which combination of available procedures is absolutely necessary for a given patient in whom joint preservation surgery is considered. Often, it is unclear which pathology is responsible for symptoms. In addition, in many patients, the appropriate treatment option is clear, but the anticipated postoperative activity goals of a patient may be unreasonable after that procedure.
Figure 4.
Intraoperative photograph of a knee with a large medial femoral condyle full-thickness articular cartilage defect showing osteochondral allograft transplantation and concomitant high tibial osteotomy.
Because patients who undergo joint preservation procedures often undergo concomitant procedures for the management of multiple underlying pathologies, interpretation of the clinical results of a single joint preservation procedure may be difficult. In addition, defining a good or excellent outcome versus a poor outcome and defining a failed procedure is a challenge because of the heterogeneity of reported outcomes. Standard patient-reported outcomes (PROs) may not adequately portray the clinical outcomes of patients who undergo cartilage restoration. For example, many surgeons may consider unplanned knee surgery after a joint preservation procedure, such as arthroscopic débridement, to be an indication of a failed joint preservation procedure, whereas the patient may be extremely satisfied with the overall outcome.[38,39] Many studies on the clinical outcomes of patients who undergo knee joint preservation surgery are retrospective in design and are subject to the weaknesses and biases inherent to level IV retrospective studies.[40] Despite these limitations, many clinical studies have been published on the outcomes of patients who undergo knee joint preservation surgery.[17,38,39,41–45] Key outcomes of patients who undergo concomitant knee joint preservation procedures are listed in Table 4.
Most knee joint preservation studies focus on the outcomes of articular cartilage restoration and MAT. In a retrospective review of 172 patients who underwent MAT that was performed by a single surgeon, 41% of whom underwent isolated MAT and 59% of whom underwent MAT and a concomitant procedure, McCormick et al[39] reported an overall allograft survival rate of 95% at a mean follow-up of 5 years, with failure defined as revision MAT or conversion to TKA. The authors reported a 32% revision surgery rate, with arthroscopic débridement being the most common procedure performed for revision surgery. Secondary surgery within 2 years of index MAT was a negative prognostic factor for failure, and patients who underwent secondary surgery had an 8.4 times odds ratio for future TKA or revision MAT.[39] Given the high incidence of procedures performed in combination with MAT in the patients in the study, it remains unclear whether the patient outcomes and revision surgery rates reported are a result of MAT alone. In a survival analysis of 180 patients who underwent osteochondral allograft transplantation (OCA), 48% of patients underwent OCA only and 52% underwent a concomitant procedure.[38] Of the patients who underwent a concomitant procedure, 36% underwent MAT. Frank et al[38]reported an overall OCA survival rate of 87% at a mean follow-up of 5 years, with failure defined as revision OCA or conversion to TKA. The authors reported a 37% revision surgery rate, with arthroscopic débridement being the most common procedure performed for revision surgery. Although most of the patients had undergone prior ipsilateral knee surgery (96%), the number of previous surgeries was predictive of revision surgery and failure.[38] The authors reported that concomitant MAT was not a risk factor for revision surgery or failure. Patients in whom revision surgery was required had substantially improved PROs; however, their outcomes were considerably inferior compared with those of patients in whom revision surgery was not required.
Getgood et al[46] conducted a survivorship analysis of 48 patients (median age, 35.8 years) who underwent concurrent MAT and OCA. Most of the patients (43 of 48) had undergone prior ipsilateral knee surgery, with a median of three procedures being performed. Bipolar (tibiofemoral) osteochondral allografts were used in 24 of the patients, and meniscal allografts were transplanted via their attachment to a compound tibial plateau osteochondral allograft in 36 patients. Failure was defined as any procedure that resulted in removal or revision of one or more of the grafts.[46] The authors reported an overall 22.9% failure rate and a 54.2% revision surgery rate, with considerable improvements in PROs reported in patients with intact grafts at a mean clinical follow-up of 6.8 years. A trend toward poorer outcomes was observed in patients who underwent bipolar tibiofemoral OCA for the management of arthritis, which suggests that earlier intervention via MAT in combination with OCA may be advantageous in patients with a less advanced disease process.[46] Overall, the results of Getgood et al[46] align with those reported in the aforementioned studies in that the overall success rate of concurrent MAT and OCA is comparable with the overall success rate of either procedure in isolation.
Patients with bipolar articular cartilage lesions who undergo joint preservation surgery have poorer outcomes than patients with unipolar articular cartilage lesions who undergo joint preservation surgery,[46] and patients with larger femoral condyle lesions who undergo joint preservation surgery have poorer outcomes than patients with smaller femoral condyle lesions who undergo joint preservation surgery. In a study of 32 patients who underwent concurrent MAT and OCA, Abrams et al[47] reported an inverse relationship between postoperative PROs and femoral condyle defect size. At a mean clinical follow-up of 4 years, considerably greater improvements in International Knee Documentation Committee (IKDC) scores, Knee Injury and Osteoarthritis Outcome Scores (KOOS), and Lysholm Knee scores were reported in the patients with a femoral condyle defect <4 cm2 compared with the patients with a femoral condyle defect >4 cm2.
Studies on the outcomes of patients who undergo realignment osteotomy in combination with MAT and/or articular cartilage restoration are limited. In a study of 18 patients (mean age, 34 years) who underwent realignment osteotomy in combination with MAT and articular cartilage restoration, most of whom had undergone prior ipsilateral surgery (mean number of previous surgeries, 2 ± 1), Harris et al[45] reported a failure rate of 11.2% and a revision surgery rate of 55.5% at a mean follow-up of 6.5 years, with considerable improvements reported in KOOS, IKDC scores, and Lysholm Knee scores. Although this study is a small series, it highlights the clinical utility of concurrent knee joint preservation procedures to fully manage all knee pathologies.
Several efforts have been made to summarize the available literature on the outcomes of patients who undergo knee joint preservation surgery. Harris et al[48] conducted a systematic review of six studies that included 110 patients who underwent MAT in combination with articular cartilage restoration (medial compartment in 66 patients, lateral compartment in 44 patients). Autologous chondrocyte implantation (ACI) was performed in 73 patients, OCA was performed in 20 patients, osteochondral autograft transfer was performed in 17 patients, and microfracture was performed in 3 patients. Additional concurrent surgery, including realignment osteotomy, cruciate or collateral ligament reconstruction, and/or hardware removal, was performed in 36 patients. Harris et al[48] reported that the clinical results of MAT in combination with cartilage restoration were similar to those of either procedure in isolation; however, revision surgery was required in >50% of patients who underwent concomitant procedures. In a systematic review of 69 studies that included 4,557 patients (mean age, 53 years) who underwent HTO with or without articular cartilage restoration and/or MAT, Harris et al[49]reported a considerably higher survivorship rate in patients who underwent HTO in combination with articular cartilage restoration (98.7%) compared with patients who underwent isolated HTO (92.4%) and patients who underwent HTO in combination with MAT (90.9%) at a follow-up of 5 years. These results are encouraging for patients in whom HTO is being considered as part of a knee joint preservation strategy.
Typically, patellofemoral joint preservation is considered separate from femoral condyle joint preservation.[50–57] Reconstructive procedures for the patellofemoral joint consist of cartilage restoration with or without realignment osteotomy. A careful evaluation of the literature is critical because patellofemoral joint reconstructive procedures can be performed with or without medial patellofemoral ligament (MPFL) repair/reconstruction for joint preservation and for the management of patellar instability. Patients with symptomatic chondral defects of the patella and/or trochlea who have normal patellar stability do not require MPFL repair/reconstruction but may require a tibial tubercle osteotomy (TTO) to offload a newly managed chondral lesion.[52] Patients with recurrent patellar instability that leads to patellar cartilage damage require cartilage restoration in combination with MPFL repair/reconstruction and TTO. In patients with a normal tibial tuberosity-trochlear groove distance, a TTO typically is performed as an anteriorization osteotomy (without medialization), whereas in patients with an abnormal tibial tuberosity-trochlear groove distance (>15 mm), a TTO typically is performed as an anteromedialization osteotomy. Therefore, surgeons must be aware of the indications for surgical management in a study if extrapolating the findings of that study to a specific patient.
In a study of 62 patients (mean age, 32 years) who underwent ACI of the patellofemoral joint, Pascual-Garrido et al[53] reported considerable postoperative improvements in most of the PRO scores, including Lysholm Knee scores, IKDC scores, KOOS, Tegner activity score, and Cincinnati Knee Rating Scale scores, at a mean follow-up of 4 years, with a trend toward better outcomes in the patients who underwent concomitant anteromedialization TTO compared with the patients who underwent isolated ACI. An overall failure rate and a revision surgery rate of 7.7% and 44%, respectively, were reported. Trinh et al[58] conducted a systematic review of 11 studies that included 366 patients (mean age, 33 years) who underwent isolated patellofemoral compartment ACI (77%) or ACI in combination with osteotomy (23%). Of the defects managed, 78% were located on the patella, and 22% were located on the trochlea. The authors reported considerable clinical improvements and a similar revision surgery rate in both groups at a mean follow-up of 4.2 years; however, in an analysis of the three studies that directly compared the outcomes of patients who underwent isolated ACI with those of patients who underwent ACI in combination with TTO, the authors reported considerably better improvements in the PRO scores of the patients who underwent ACI in combination with TTO.[58]
In a multicenter study of 110 patients who underwent ACI in the patella, 75 of whom underwent concomitant realignment osteotomy, Gomoll et al[52]reported statistically significant and clinically relevant improvements in pain and function in all PRO scores at a mean follow-up of 7.5 years and that 92% of patients stated they would undergo the procedure again. The authors reported an overall failure rate of 8%, with no considerable differences reported in the outcomes or failure rate of the patients who underwent TTO in combination with ACI and those of the patients who underwent isolated ACI; however, the TTO and ACI group included a substantially greater number of patients than the ACI-only group, which suggests that the study was underpowered to detect such a difference.[52] In addition, most of the patellar lesions in the study were medial or central lesions (87%) rather than lateral lesions, which may respond more favorably to TTO. The authors highlighted the importance of preoperative planning and understanding surgical indications in patients who undergo cartilage restoration to determine if concomitant realignment procedures are appropriate.
Recently, the use of biologics for the management of articular cartilage lesions has increased considerably. Although scientific and clinical evidence on biologic therapy is evolving, biologics may help prevent articular cartilage lesion progression and may play a role in the nonsurgical and surgical management of osteoarthritis and focal chondral lesions.[59–61] Although knee joint preservation surgery is effective in appropriately indicated patients, revision surgery rates are relatively high, and failure rates increase with longer follow-up. Given the relatively young age of most patients who undergo knee joint preservation surgery, efforts to improve the short-term function and long-term duration of knee joint preservation surgery are under way, with a major focus on biologic augmentation. In general, biologic agents are believed to inhibit inflammation and promote tissue healing. The biologic agents most frequently used for articular cartilage and meniscal management include platelet-rich plasma (PRP), mesenchymal stem cells (MSCs), and biologic scaffolds. Research has been done to attempt to evaluate the effect of these biologic agents and growth factors, such as transforming growth factor-[beta]1, bone morphogenetic protein 7, and insulin-like growth factor 1, all of which have been reported to increase chondrocyte synthetic activity in in vitro studies.[62]
PRP, which is an autologous, highly concentrated product containing growth factors and inflammatory mediators, has been reported to enhance chondrocyte proliferation, and ongoing studies are attempting to evaluate the differentiation and functionality of those chondrocytes.[61,63,64] PRP is an autologous product that is produced by harvesting peripheral blood via standard venipuncture techniques, spinning the peripheral blood in a centrifuge to concentrate platelets above baseline levels, and injecting the finished product into an affected area. Platelets are of interest because they contain a variety of growth factors that are known to stimulate the proliferation of local progenitors, direct cell differentiation, and modify inflammatory responses.[59,65] Considerable variation exists with regard to the manner in which PRP is harvested and spun,[66–71] which makes comparison of the results of PRP in one study with those in another study almost impossible.
A variety of factors, including donor-related variables (eg, age, sex, nutritional status), processing-related variables (eg, collection and storage conditions, spin protocol, activation agent), and delivery-related variables (eg, delivery vehicle, timing of delivery relative to harvest, injury chronicity), influence the growth-factor profile of PRP.[59,66,67] The volume of leukocytes in PRP is one factor of considerable interest.[71,72] PRP is classified as leukocyte-rich or leukocyte-poor, depending on its final leukocyte concentration before injection. Leukocytes are present in the buffy coat layer, which often is merged with the platelet-rich portion.[73] Because PRP preparation protocols are not standardized, PRP preparation may or may not separate the buffy coat layer from the platelet-rich portion.[74,75] The differences between leukocyte-rich PRP and leukocyte-poor PRP are not completely understood, especially with regard to treatment indications. Several preclinical studies reported that leukocyte-poor PRP may be better suited for intra-articular use than leukocyte-rich PRP.[71,72] In a prospective, double-blind, controlled clinical trial of 111 patients with mild to moderate knee osteoarthritis who were randomized to intra-articular injections of leukocyte-poor PRP or hyaluronic acid, Cole et al[76] reported no differences in Western Ontario and McMaster Universities Osteoarthritis Index pain subscores between the patients in the two groups; however, several other outcome measures favored PRP more than hyaluronic acid. Although the study was performed in patients with knee osteoarthritis, the results may be translatable to patients with focal chondral or osteochondral defects. Additional research is necessary to determine the long-term clinical effects of PRP on knee cartilage pathology, including osteoarthritis.
In general, stem cells are classified as embryonic stem cells, induced pluripotent stem cells, or adult stem cells.[61] Stem cells are subclassified as autologous or allogeneic. Autologous adult MSCs are mostly harvested from bone marrow and adipose tissue. Allogeneic stem cells can be harvested from placental and amniotic tissues. The advantages and disadvantages of stem cells used for knee joint preservation are listed in Table 5. In a prospective, single-blind, placebo-controlled trial of 25 patients with bilateral knee pain attributed to bilateral osteoarthritis who were randomized to receive an intra-articular injection of iliac-crest–derived bone marrow aspirate concentrate (BMAC) in one knee and saline placebo in the contralateral knee, Shapiro et al[77] reported similar outcomes with regard to pain relief in both the BMAC-treated and the placebo-treated knees 1 week, 3 months, and 6 months postinjection.
The use of scaffolds to augment knee joint preservation surgery has been more consistently described in the literature compared with the use of PRP or stem cells, mainly because scaffolds are used during advanced ACI (matrix-induced ACI, matrix-assisted ACI) for the management of articular cartilage lesions.[54,78–82] Meniscal repair and replacement with the use of scaffold-based technology has been described in Europe, with encouraging short-term results reported.[83–92] In general, scaffolds are categorized based on whether they incorporate cells and whether they are synthetic or biologic in origin.[93] Other important features of scaffolds include their mechanical properties and the type of tissue they target (ie, articular cartilage, meniscus, ligamentous tissue). Scaffold materials used for cartilage restoration include protein polymers (ie, collagen and fibrin), carbohydrate polymers (ie, hyaluronic acid), synthetic polymers, and polymer composites.[94]
Interpretation of the literature on the use of PRP, stem cells, and scaffolds to augment knee joint preservation surgery is a challenge because of the paucity of studies with midterm to long-term follow-up and the heterogeneity of the agents used. Even among studies that analyzed the same agent, such as PRP, preparations may differ, which makes the interpretation of results a challenge.[67,69,71,95] With regard to intra-articular knee pathology, PRP has been used for the nonsurgical, injection-based management of knee pain that is attributed to a variety of etiologies but mainly osteoarthritis. In addition, PRP has been used to augment the surgical management of articular cartilage defects, with encouraging preclinical results reported.[66,96,97] For this augmented procedure, a commercially available biologic scaffold of micronized allograft articular cartilage matrix is augmented with PRP and placed in the defect bed after microfracture to induce type II hyaline cartilage rather than the type I fibrocartilage typically produced via traditional marrow stimulation techniques.
Recent studies on allogeneic and autologous stem cells for the management of articular cartilage defects and osteoarthritis have reported acceptable safety profiles and encouraging short-term results. In a small safety study of six patients with knee osteoarthritis who received a single intra-articular injection of cryopreserved particulated human amnion and amniotic fluid cells, Vines et al[98] reported considerably improved PROs, including KOOS, IKDC scores, and Single Assessment Numeric Evaluation scores, and considerably increased serum immunoglobulin G and immunoglobulin E levels at a follow-up of 12 months.
Other studies have assessed the effect of adipose-derived MSCs in the management of focal chondral lesions of the knee and generalized knee osteoarthritis. In a controlled trial of 80 patients who were randomized to microfracture with or without adipose-derived MSC augmentation (covered with fibrin glue), Koh et al[99] reported complete cartilage lesion coverage in 65% of the patients in the MSC group and in 45% in the microfracture-only group based on MRI obtained at follow-up of 2 years. In addition, improvements in mean KOOS pain and symptom subscores were considerably greater in the patients in the MSC group compared with the patients in the microfracture-only group at a mean follow-up of 27.4 months; however, no substantial differences in the other KOOS subscores were reported between the patients in the two groups. Similar encouraging outcomes have been reported in older patients with diffuse knee osteoarthritis who undergo treatment with the use of adipose-derived MSCs during knee arthroscopy.[100,101] Perdisa et al[102] published a systematic review that summarized the evidence supporting adipose-derived MSCs for the management of articular cartilage disease. The authors reviewed 39 studies, including 28 animal studies and 11 clinical studies. Of the 11 human clinical studies, the authors reported that the methodologies varied, with MSC harvest locations including the buttocks, the abdominal area, and the infrapatellar fat pad and with administration protocols varying from a single injection of 3 to 5 mL with or without PRP (or hyaluronic acid) to a single injection of 3 to 5 mL with or without PRP (or hyaluronic acid) after arthroscopic débridement. Follow-up duration also varied, ranging from 3 to 36 months. Overall, most of the studies reported improvements in pain and functional scores.[102] Although these results are encouraging, they must be interpreted with caution given the low overall number of patients, the heterogeneity of methodology, and the lack of consistent follow-up.
The use of BMAC as a biologic solution for knee joint preservation also has been recently described[103–107] (Figure 5). Although BMAC contains a relatively low concentration of stem cells, it is believed to contain numerous growth factors that are important because of their anabolic and anti-inflammatory properties.[103] In a recent systematic review of 11 studies on the clinical efficacy of BMAC in the management of knee cartilage pathology (three studies on the management of osteoarthritis, eight studies on the management of focal cartilage injuries), Chahla et al[103] reported good to excellent outcomes in all of the studies. In a nonrandomized study of 37 patients with patellofemoral chondral lesions who were treated with BMAC or matrix-induced ACI, Gobbi et al[104] reported considerable improvements in the PROs of the patients in both groups at a minimum follow-up of 3 years, with no significant differences in improvement with regard to most PROs between the patients in the two groups; however, higher IKDC subjective scores were reported in the patients in the BMAC group (P = 0.015).
Intraoperative photograph of a knee showing the technique for harvest of bone marrow aspirate concentrate from the posterior iliac crest.
Allograft tissue is considered a biologic treatment option for patients who undergo knee joint preservation. In addition to fresh osteochondral and meniscal allografts, other allograft products include particulated juvenile cartilage allograft tissue; three-dimensional osteochondral allograft matrices; and cryopreserved osteochondral allografts that contain native viable chondrocytes, growth factors, and extracellular proteins (Figure 6). These surface allografts are commercially available off the shelf and may be a viable alternative to fresh osteochondral allografts and osteochondral autograft; however, surface allograft transplantation should be considered only in patients with normal or near-normal subchondral bone. Although encouraging early clinical outcomes have been reported in patients who undergo particulated juvenile cartilage allograft transplantation for the management of focal chondral defects,[108–113] no clinical studies are available on the outcomes of patients who undergo treatment with the use of other off-the-shelf surface allografts.
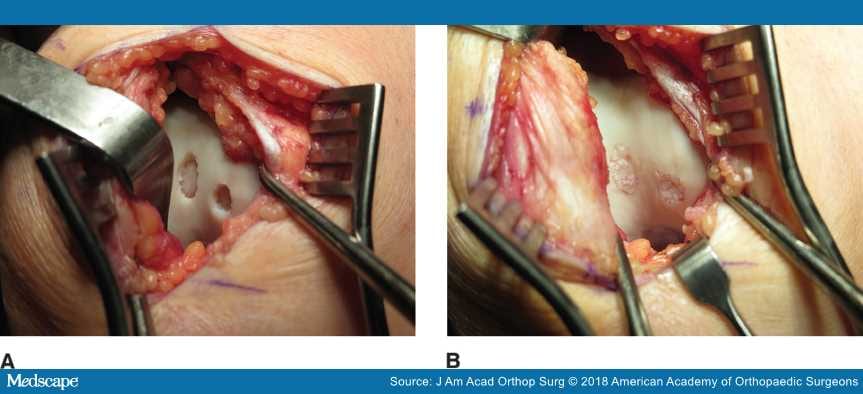
Figure 6.
Intraoperative photographs of a knee showing the appearance of two small trochlear chondral lesions before (A) and after (B) particulated juvenile cartilage allograft transplantation.
Although the number of clinical trials on MSCs and PRP has increased substantially in the past decade, many early studies were conducted before underlying disease processes and therapeutic mechanisms were completely understood.[109,110] Given the increased interest in PRP, MSCs, scaffolds, and other cell- and tissue-based products, industry, orthopaedic surgeons, and regulatory agencies must collaborate to conduct efficient, high-quality research.[59,60,108] Early data on PRP, MSCs, and other biologics are promising; however, additional studies are necessary to further elucidate the mechanisms via which these biologics exert their effects so that their ultimate potential can be realized in patient care.
Patients with concomitant knee pathologies, including articular cartilage defects, meniscal deficiency, ligamentous insufficiency, and/or malalignment, are the patients who are most difficult to treat via joint preservation. Clinical decision making for these patients is a challenge, particularly because most patients are young and have expectations to return to high-demand activity. The current literature supports multiple knee joint preservation strategies, including realignment osteotomy, if indicated. In the past decade, the number of clinical studies on biologic therapy has increased exponentially, with most studies reporting acceptable safety profiles and encouraging short-term results. Therefore, the use of biologic therapy as an alternative to or to augment more conventional knee joint preservation techniques is likely to increase. Additional long-term studies are necessary to determine the efficacy and cost-effectiveness of knee joint preservation techniques.