Results
Patient Characteristics
Overall, 607 of 892 eligible participants were enrolled into the study: 304 in the randomized cohort and 303 in the observational cohort (Figure 1). Of these, 601 completed at least one follow-up visit. A total of 406 surgery patients were available at 8 years post enrollment, constituting 69% of the randomized and 57% of the observational participants.
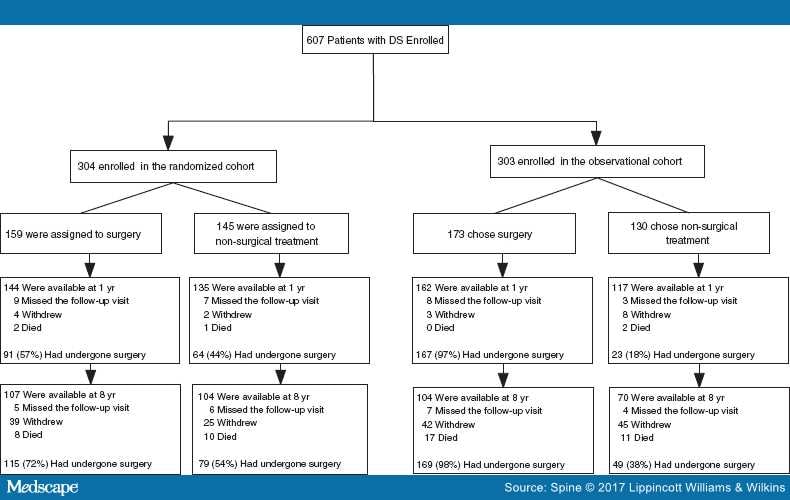
Figure 1.
Flow diagram of the Spine Patient Outcomes Research Trial degenerative spondylolisthesis (DS) study: randomized and observational cohorts. Numbers reported at follow-up time points are cumulative and percentages are calculated from the number of patients in each respective arm.
Univariate analysis revealed patients who had undergone a reoperation by 8 years were younger than those without a reoperation (62.2 vs. 65.3 years, P= 0.008). Patients who had a reoperation were less likely to have neurogenic claudication at baseline (73% vs. 89%, P < 0.002). There were no significant differences in other demographic variables or specific comorbidities (Table 1). Patients who underwent a reoperation had worse SF-36 BP (28.1 vs. 32.6, P= 0.044), ODI (47.0 vs. 43.1, P = 0.048), and Stenosis Frequency Index scores (15.8 vs. 14.3, P = 0.021) at baseline.
Operative Outcomes
Univariate analysis revealed patients in the reoperation group had a higher incidence of total postoperative complications following the index surgery (39% vs. 27%, P = 0.036). However, there were no significant differences in wound hematoma rate, infection rate, neurological injury, or dural tear between the reoperation and nonreoperation groups. There were no significant differences in other operative outcomes measures including type of operation, operative time, blood loss, length of hospital stay, or incidence of intraoperative complications (Table 2).
When comparing all patients included in the study, the noninstrumented fusion group had a higher rate of decompression at L2-L3 (20% vs. 10%, P = 0.014) and L3-L4 (61% vs. 46%, P = 0.022) than the instrumented fusion group. The noninstrumented fusion group had a significantly shorter operative time (157.6 vs. 226.6 minutes, P < 0.001), less blood loss (506.5 vs.632.2, P = 0.031), and a lower rate of intraoperative blood replacement (27% vs. 38%, P = 0.062) than the instrumented fusion group (Table 3). There was no significant difference in the rate of wound hematoma, neurological injury, or infection between instrumented and noninstrumented cases.
Surgical Treatment
Of the 406 surgery patients, 72% underwent instrumented fusion, 21% noninstrumented fusion, and 7% underwent decompression alone; 97 underwent a multilevel fusion (24%). At 8-year follow-up, 91 of 406 (22%) had a reoperation. Of these patients, 25 occurred within the first year (28%), 49 within 2 years (54%), 64 within 4 years (70%), and 78 within 6 years (86%) (Table 1 ; Figure 1). The reasons for reoperation included recurrent stenosis or progressive spondylolisthesis (45%), complications such as hematoma, dehiscence, or infection (complete list in Table 3) (36%), and the development of a new condition (14%).
Patient-reported Outcomes
Patient-reported outcomes at 8 years were compared between the reoperation and no reoperation groups. For both primary and secondary outcomes (including SF-36 BP, SF-36 PF, ODI, SBI, and satisfaction with symptoms), patients in the reoperation group had significantly less improvement, represented by mean change in scores from baseline to 8-year follow up, (Table 4; Figure 2).
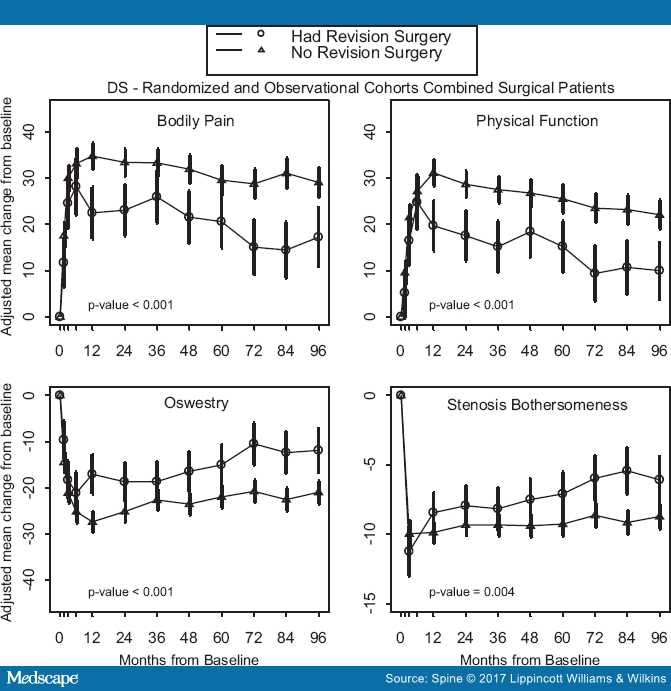
Figure 2.
Primary outcomes over time with area under curve P value that compares average 8-year mean change from baseline between reoperation and no reoperation groups.
Risk Factors for Reoperation
Multivariate regression analysis using all of the baseline demographic variables in Table 1 showed that age (P = 0.018, hazard ratio [HR] 0.97); patients with 2/3 moderate or severe stenotic levels (P = 0.02, HR 1.71); predominant back pain (P = 0.023, HR 2.09); no physical therapy (P = 0.017, HR 1.74); absence of neurogenic claudication (P = 0.019, HR 1.89); and greater leg pain score at baseline (P = 0.003, HR 1.39) predicted higher reoperation rates (Table 5). Factors that did not affect the risk for reoperation included: smoking, diabetes, obesity, longer duration of symptoms, or workman's compensation.
Of the patients who underwent reoperation, 80 of 91 patients had complete information available on reoperation levels. Eighteen of 80 (22.5%) patients had a reoperation at the same level as the index operation, 40 of 80 (50%) had a reoperation of at least one different level than the index operation, and 37 of 80 (46%) had levels unspecified. Of these 80 patients, 13 patients had two reoperations and one had three reoperations (data not shown).
Multivariate analysis was used to further investigate the demographic risk factors for reoperation for patients who received noninstrumented fusion or instrumented fusion. Using all of the demographic factors in Table 1, in the noninstrumented fusion group, patients without neurogenic claudication were more likely to undergo reoperation (P = 0.0034, HR 7.12), as were patients of older age (P = 0.009, HR 0.92). In the instrumented fusion group, patients without neurogenic claudication (P = 0.0098, HR 2.16) and those with a higher baseline SBI score (P = 0.0161, HR 1.06) were more likely to undergo reoperation (data not shown).
Discussion
This study performed a subanalysis of the 8-year SPORT data to determine baseline risk factors for reoperation in patients treated surgically for DS, and compare outcomes with patients that did not undergo reoperation. Several similar studies have been limited by small demographic groups, homogenous cohorts, or by retrospective analysis.[7,10–13] This is the first subanalysis of its kind using SPORT data.
The incidence of reoperation for patients treated surgically for DS was 22% at 8 years, with 54% of these occurring in the first 2 years. The most common indication for reoperation was progressive spondylolisthesis or recurrent stenosis (45%). These findings are consistent with several reports in the literature with similar patient demographics.[1–3,5,7,10,11,13]
Previously reported risk factors for adjacent segment disease and radiographic degeneration after lumbar fusion include age,[10,11,13] number of levels fused,[9,10,12] preexisting disc degeneration,[14,15] smoking,[16] BMI,[11]and preexisting facet degeneration.[8] The strongest baseline positive predictors for reoperation in this study were lack of neurogenic claudication and back pain predominant symptoms (HR = 1.89, HR = 2.09, respectively). Interestingly, demographic factors including smoking, obesity, diabetes, duration of symptoms, and workman's compensation were not associated with an increased risk of reoperation.[10] Similarly, surgical factors such as the type of surgery performed, blood loss, time of operation, intraoperative complications, level of fusion, and multilevel laminectomy were not associated with an increased risk for reoperation.
Compared to patients with neurogenic claudication, patients without neurogenic claudication who underwent fusion showed a higher risk for reoperation. They were seven times as likely to undergo reoperation with noninstrumented fusion and twice as likely to undergo reoperation after instrumented fusion. Further studies are needed to address this question, but the findings suggest that fusion with instrumentation may result in fewer reoperations in patients without neurogenic claudication.
Patients who had a reoperation were more likely to have a history of antidepressant use (11% vs. 4%, P < 0.014). However, depression itself was not a significant risk factor. Although other studies have identified the correlation between inferior outcomes and depression for patients undergoing lumbar spine surgery, they have not reported on outcomes in patients taking antidepressants without underlying depression.[1,2,8,16,17] This may indicate that patients with DS on antidepressants for treatment of chronic pain are predisposed to worse clinical outcomes.
Contrary to previous studies, the overall use of instrumentation showed no significant difference in reoperation rates at 8-year follow-up (21% vs. 24%, P= 0.51)[1,2,5,7,11,17] Also, rates of late complications requiring reoperation, (progressive spondylolisthesis, recurrent stenosis, pseudoarthrosis, wound exploration, or other complications) were not significantly different between these two groups (P = 0.60). This is contrary to several reports that suggest instrumented fusion may facilitate the development of complications such as adjacent segment disease that may require a reoperation.[9,13,17–20]
In this analysis, there was a surprisingly low pseudoarthrosis reoperation rate (1%–2%, Table 2) among the entire SPORT DS cohort who underwent fusion. However, patients in the SPORT trial were not routinely imaged during follow-up; thus, pseudoarthrosis rates, particularly those that were asymptomatic, may be underreported. Kornblum et al[18] noted that patients with a solid fusion have significantly better outcomes than patients who develop a pseudoarthosis. Subsequent articles have established no difference in outcome because of fusion technique or use of instrumentation.[17,19] The discrepancy between the present study pseudoarthrosis rate and the historical pseudoarthrosis rate may lie in the fact that this represents the symptomatic pseudoarthrosis rate, which may be different from the radiographic pseudoarthrosis rate, which was evaluated in previous studies. Similar to other studies, number of levels fused was not associated with increased risk of reoperation.[21]
The SF-36 BP and PF, ODI, SBI, and satisfaction with symptoms showed significantly greater improvement in the no reoperation group. These findings are consistent with other reports showing worse outcomes in patients requiring a reoperation.[7,10,11,13] Although both groups of patients improved from baseline, our data indicate that reoperation may not be able to "rescue" the symptoms of an unsuccessful initial surgery.
Martin et al studied reoperation rates in a large retrospective study (n = 24,882) and found patients with spondylolisthesis had a lower reoperation rate after fusion surgery than after decompression alone (17.1% vs. 28%, P = 0.002).[7,13] In a similar study, Lad et al[14] found higher complication rates in patients undergoing arthrodesis compared with decompression alone (8.3% vs. 4.8%; P < 0.0001). These findings were similar to our study, although both of these studies included heterogenous patient groups.
Limitations
The drop-out rate with any long-term study can also confound the study findings. Patients with worse outcomes may seek treatment elsewhere or not at all, and patients doing well following surgical or nonsurgical intervention may also be less likely to follow-up. Reasons for reoperation are unknown other than broad categories such as recurrent stenosis, progressive spondylolisthesis, infection, or pseudoarthrosis. We do not have information on the specific symptoms or findings that prompted reoperation. Another possible limitation of this study is limited sample size for subgroup analyses, which could limit the ability to identify a significant factor associated with reoperation. However, this study comprises one of the largest series in the literature on a pure population with DS with good follow-up. Finally, radiographic parameters such as sagittal alignment and pseudarthrosis may influence the rate of adjacent and same segment disease,[21,22] yet the limited available radiographic data precluded our ability to include these radiographic analyses.
Conclusion
Overall, this study demonstrated a modest reoperation rate of 22% at the 8-year follow-up for patients treated surgically in the DS arm of the SPORT trial. Reoperations were most commonly because of recurrent stenosis or progressive spondylolisthesis. The strongest predictors for reoperation were patients with predominant back pain symptoms and patients without neurogenic claudication at enrollment. As patients are guided through the shared decision-making process, these risk factors and the understanding of patient-specific outcomes can facilitate making the best decisions between physicians and patients. Future studies should involve larger prospective studies of patient subgroups with DS to improve the individualization of treatment in the clinical setting.
