A high prevalence of PA following a DRF in young non-osteoporotic patients was found (32%). Patients with PA had statistically significant longer radial length than patients without PA. Within the group of patients with PA, radial length was also longer in comparison to the uninjured wrist. Patients healed with a residual gap more often had PA. PA was associated with diminished flexion/ extension arc of motion and ulnar/radial deviation arc of motion. Patients healed with a residual gap more often had PA. When corrected for dominance, no statistically significant differences in grip strength measurements between patients with and without PA are present. In patients with PA the subscales ‘general functioning’, ‘esthetics’ and ‘satisfaction’ from the MHQ questionnaire were statistically significant poorer, as was the total MHQ score and the physical functioning scale of the SF-36. The DASH, PRWE function and PRWE total were statistically significant impacted by flexion/extension arc of motion.
Prevalence of PA
The high prevalence of PA of 32% after a median follow-up of 5 years in this young population was surprising. Forward et al. presented a prevalence of 43% after a mean follow up of 38 years in non-osteoporotic patients at time of the injury [4]. The prevalence in our study might be overestimated due to the low response rate, as individuals with complaints might be more interested in participating in research activities. This assumption is supported by the fact that participants had sustained more intra-articular DRFs than non-participants. Further research on the prevalence of PA after DRFs in young patients is needed.
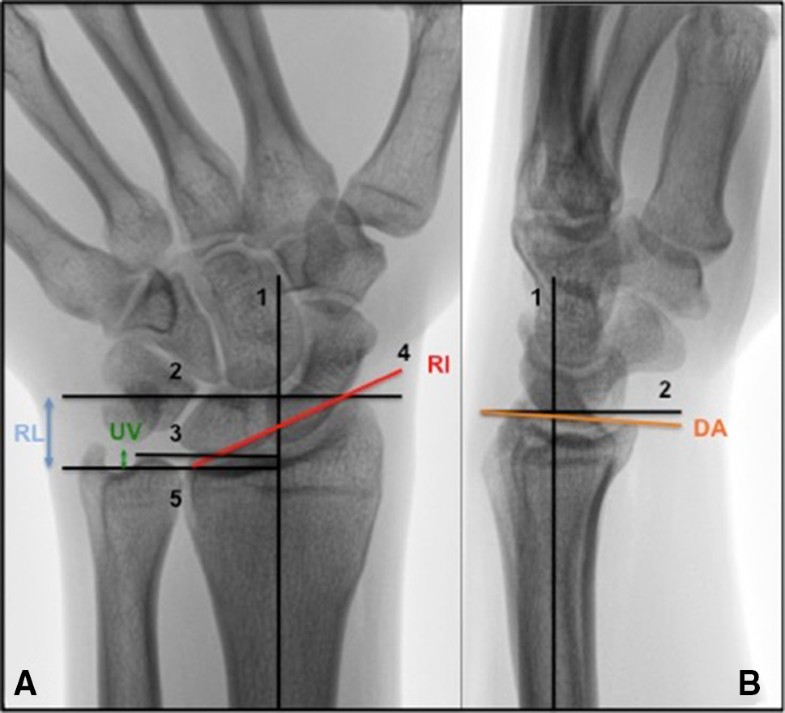
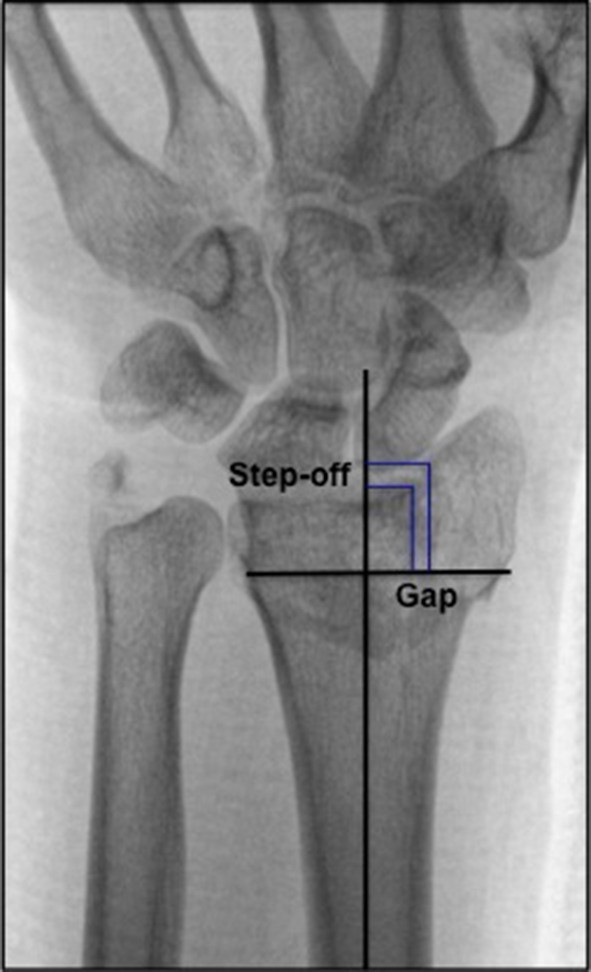
Radiological measurements
DRFs in non-osteoporotic patients mainly result from high-energy trauma and therefore frequently lead to intra-articular fractures [47]. Our results are supported by literature, suggesting that DRFs that healed with a residual gap and/or overall intra-articular incongruence of ≥ 2 mm are associated with early radiographic signs of PA [3, 4, 18, 48, 49]. In addition, a systematic review recently published by our research group established that other radiological predicting factors for PA such as radial length, radial inclination, dorsal angulation and ulnar variance are presented in literature with conflicting results [3]. Regarding radiological measurements, only radial length was 1.1 mm longer at follow up in patients with PA in comparison to patients without PA. All studies reporting on the influence of radial length, reported on shortening of radial length. Most studies reported no statistically significant association with shortened radial length and the development of PA [1, 5, 50], except for Forward et al. [4]. The development of PA has multifactorial causes, such as increased stress on the articular surface that damages cells and matrices of articular cartilage and subchondral bone [2]. Overcorrection of the radial length can cause higher axial loading on the articular surface of the distal radius and therefore may contribute to the development of PA [51, 52]. In previous literature normal ranges for radiological factors have been described; ulnar variance − 4 to 2 mm [31, 32], radial length 8–17 mm [33], radial inclination 16°–29° [31, 34], dorsal angulation 0-palmar 22° [35, 36]. All measurements in this study were within these normal ranges. Although radial length seems to influence the development of PA, more research regarding these radiological predicting factors for PA is mandatory to provide constructive conclusions. Common held beliefs are dictated by the findings stated earlier that anatomical reduction of articular surfaces and absolute stable internal fixation should be pursued. In this study, no statistically significant difference regarding presence of PA between patients who were treated conservatively and operatively was found. This could suggest that anatomical reduction was achieved when patients were treated surgically and little residual incongruence was present following treatment. The relatively short follow-up period (5 years) in our study should preferably be extended in further research to get insight in the ‘natural’ course of PA in young patients with DRF. With regard to CROs, it has been reported that presence of PA did not influence aROM and grip strength after 15 year follow up [53]. However, the impact of PA after long term follow up on participation in societal roles and in the personal lives of these young people, captured with PROs remains unclear.
CROs
Patients with PA showed a diminished flexion/extension arc and ulnar/radial deviation arc of motion. It is known that residual articular incongruence affects aROM already after a follow up of 1 year [54]. Other authors have shown that patients can maintain a high level of functioning with PA [53]. Articular incongruency is the logical result from intra-articular fractures, however in our study no statistically significant association between fracture severity as depicted by the AO/OTA classification and active range of motion was found. In addition, associated intercarpal ligamentous injuries are known to influence active range of motion following DRFs and could be an explanation for the diminished active range of motion found in our study [12, 55].
Grip strength and key pinch strength of the injured side compared to the uninjured side seemed to be affected by PA. However, when correcting for dominance with the 10% rule, this statistically significant difference resides [37]. This finding supports literature emphasizing that grip strength is not a determinant of wrist function alone, but merely a reflection of overall muscle strength and condition of a chain of muscles in the upper limb [56]. We do believe that measurement of differences in grip strength between injured and uninjured side are relevant when determining follow-up outcomes of patients who sustained a DRF. Minimal detectable change (MDC) is defined as the smallest amount of change between two measurements that indicates a real change in measurement and not being a change due to measurement error [57]. This is a statistical measurement and does not take into account change as experienced by patients. Minimal clinically important difference (MCID) is the smallest change in a measurement that a patient would notice [57]. For grip strength MDC has been reported to be 6.5 kg and MCID 19.5% or 6.5 kg in patients 1 year following surgery for DRFs [58]. This suggests that the grip strength measurements between patients with and without PA presented in our study after a median follow up duration of 5.2 years are not noticeable for patients and therefore are possibly not clinically relevant. However, since PA is a chronic, progressive disorder, grip strength differences may become clinically relevant after a longer follow-up time. Further research is needed to provide more insight in this issue.
PROs
Pain did not differ statistically significant between patients with or without PA suggesting that pain may not be the main problem non-osteoporotic patients are facing following a DRF. In patients suffering from hand osteoarthritis pain intensity does not correlate strongly with radiographic classification [59]. The fact that the level of pain was similar in both groups might be explained by the relatively short follow-up period. However, the follow up duration was long enough to show a significantly decreased active range of motion in patients with PA compared to patients without PA. This suggests that evaluation of young patients with DRFs should not only be guided by pain but also by other domains.
Another interesting finding of the application of the different PROs was that performance of activities in daily life and work, as measured by the DASH and PRWE, questionnaires specifically designed for upper extremity functioning, was similar in patients with PA compared to those without PA. In contrast, general functioning, esthetics and satisfaction as measured by the MHQ subscales were statistically significant lower in patients with PA, as was the subscale physical functioning in the SF-36 and the total MHQ score. For future research these findings imply that other dimensions, different from those measured by the commonly used PRWE and DASH should be evaluated when measuring consequences of PA in patients with DRFs. MDC and MCID have been described to be respectively 7.7 and 17.3 for PRWE and 9.3 and 13.8 points for DASH [60]. For the MHQ none of the domains were reported to be discriminative after 3 years following volar plate fixation for DRFs, but for patients with carpal tunnel syndrome, MCIDs of 23, 13 and 8 were identified for the pain, function and work domains, respectively [61]. This suggests that the difference reported in this study in general function domain between patients with and without PA (10.0, p = 0.018) might not be relevant for patients. However, the study by Shauver et al. described 12 points difference on the satisfaction domain to differentiate between satisfied and unsatisfied patients. Therefore, the difference between patients with and without PA regarding the satisfaction domain (16.7, p = 0.044) might be clinically relevant. Unfortunately, no MDC or MCID have been described for the domains of the SF-36. It is surprising that the more subjective domains such as esthetics and satisfaction were statistically significant associated with PA and not the domains reporting on pain and (daily) functioning. The impact of PA for patients following DRFs in everyday life, while there is limited aROM, does not seem to be significant. However patients with PA are less satisfied. Further research should clarify the specific reasons for dissatisfaction.
Waljee et al. recently described a core set of domains that should be reported in order to get insight into outcomes after a DRF for clinical or research purposes: performance, PROs, pain, complications and radiographs [62]. Domains found in our study to be statistically significant different between participants with and without PA such as satisfaction and esthetics, are however lacking in this core set. To report about patient satisfaction is becoming increasingly important, since in modern medicine patient-centered health care is emphasized. Decision making in healthcare has shifted from a paternalistic model to informed decision-making and shared decision-making. There is evidence suggesting that shared decision-making does facilitate positive health outcomes and improves satisfaction [63]. In the future, it might be beneficial to further explore which elements of Waljee’s proposed core set are relevant in the clinical follow up of non-osteoporotic patients following DRFs [62, 64]. With regard to the PROs, we recommend the use of the MHQ subscales in clinical practice and post-injury DRF research, as this instrument seems to distinguish between patients with or without PA in the univariate analyses.
In literature, the few studies reporting on associations between CROs and PROs following DRFs describe these results at short-term follow up [65, 66, 67, 68]. Chung et al. use two questions of the MHQ subscale satisfaction regarding range of motion and grip strength to determine cut-off points for satisfaction 3 month following a DRF. Optimal cut-points to distinguish satisfaction from dissatisfaction were met when patients recovered 65% of their grip strength and 95% of the wrist arc of motion [65]. Shauver et al. describe linear regression analyses revealing that 3 months following a DRF, patient’s education, income, age at time of surgery and all measured outcome variables (grip strength difference, pinch strength difference, flexion, extension, active arc of motion, ulnar deviation, radial deviation, pronation, supination) accounted for 37% of the explained variance in total MHQ score [66]. Souer et al. describe a model where independent predictors pain (F = 61.16, p < 0.001) and forearm rotation (F = 27.39, p < 0.001) account for 71% of the explained variance of the DASH at a median follow-up of 6 months [68]. Our results support the finding that especially active range of motion is an important determinant of PROs. Type of treatment and AO/OTA fracture type did not seem to influence PROs. In addition, the influence of predicting factors seems to become less prominent with a longer follow-up duration as the explained variance in our study was lower than the earlier mentioned studies [66, 68]. Future research should be aimed at determining a complete overview of factors influencing PROs following DRFs in non-osteoporotic patients at early, but also at longer follow-up period. From our study, we conclude that the development of PA impacts active range of motion. Patients perceive diminished general functioning and satisfaction following PA and diminished active range of motion. This insight could direct rehabilitation strategies and can be used to counsel these patients on expected outcome.
Our results reporting 10% change of occupation following a DRF are likely to be of major interest for patients. No statistically significant association with PA was found. Although all patients reported to have changed occupation because of the injury, this percentage might be a normal change of occupation in this population. It is striking however that all patients changed to a physically less demanding occupation and change of occupation occurred more often in physically demanding jobs. This suggests that patients more often need to adapt their working environment following a DRF when having a physically demanding occupation.
Strengths and weaknesses
Where most studies report on osteoporotic patients, sometimes combined with non-osteoporotic patients, we report on a young non-osteoporotic population who sustained a DRF 4–11 years ago. As such, we contribute to the knowledge PA and its association with radiological measurements, CROs and PROs in young patients. In addition, we have used measurements of the uninjured wrist as control when calculating the percentage of grip strength. Large variations between patients are accounted for in this way. The active range of motion and grip strength measurements were performed by one hand therapist for consistency. Measurements were performed in a fixed sequence. As a consequence however, fatigue effects may have influenced our results. In future research, a random sequence of measurements should be considered. Intraobserver and interobserver variability of radiological measurements and AO/OTA fracture classifications of DRFs on radiographs is known to be moderate [29, 69]. To eliminate interobserver variability, all measurements on radiographs were performed by one specialized radiologist. It has to be acknowledged that, although all radiographs have been performed according to protocol, measurement accuracy can be influenced by the quality of the radiograph taken and computed tomography could be more sensitive. The patients in our study did not have radiographic measurements out of normal ranges as described in literature [31, 32, 33, 34, 35, 36]. Still, a prevalence of 32% PA at a relatively short follow up duration of 5 years is a substantial portion and is likely to progress with longer follow up duration. Our response rate was low, presumably because this population is young and has moved for study or work purposes and therefore many current addresses could not be retrieved. The included number of 73 patients might be insufficient to draw firm conclusions. However, in most studies describing populations after DRF the number of patients included in this study is not exceeded [10, 65, 70, 71]. Moreover those studies do not report response rates [4, 18, 20, 48]. Our results contribute to the knowledge on how to improve outcome and diminish PA in the future. However, studies with longer follow up duration are mandatory to gain more insight in the influence of progressed PA on outcome.