Low back pain (LBP) resulting from degenerative lumbar spine disease is a leading contributor to global disability. Changes in the morphology of the lumbar multifidus muscle on magnetic-resonance imaging (MRI) are associated with worse LBP and disability, but the association between multifidus morphology and post-operative outcomes is not known. The purpose of this systematic review is to examine the relationship between pre-operative multifidus morphology and post-operative changes in pain and disability.
We performed a systematic search using the Cochrane Library, EMBASE, MEDLINE, CINAHL and Scopus databases covering the period from January 1946 to January 2018. The literature was searched and assessed by independent reviewers according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement. All relevant papers were assessed for risk of bias according to the Quality in Prognosis Studies tool.
The initial search yielded 436 studies, of which 6 studies were included in the analysis. Four studies were at a low risk of bias. These studies included a total of 873 patients undergoing spinal surgery. An association between low fat infiltration and greater improvement in LBP and disability following surgery was identified. There was insufficient evidence to identify a relationship between cross-sectional area (CSA) and LBP or disability.
This systematic review found evidence for an association between low multifidus fat infiltration on MRI at baseline and greater reductions in measures of LBP and disability following surgical treatment. There is also limited evidence for an association between larger pre-operative multifidus CSA and improvements in disability, but not pain. The findings of this review should be interpreted with caution due to the small quantity of the available literature.
Low back pain (LBP) is a common and debilitating health problem. It is the leading contributor to global disability, and up to 80% of the population will experience at least one episode in their lifetime [1, 2].
Despite its prevalence, the aetiology of most LBP is uncertain. Trauma, malignancy, infection and other systemic diseases account for approximately 15% of instances, with the majority of remaining cases having no specific cause [3]. A meta-analysis by Endean et al. [4] found that disc degeneration and protrusion, nerve root compression and high-intensity zones on magnetic-resonance imaging (MRI) are reliably associated with non-specific LBP. Additionally, recent evidence suggests that multifidus muscle morphology on MRI is associated with LBP. The multifidus acts to both rotate and stabilise the lumbar spine. Altered morphology, specifically a smaller functional cross-sectional area, in the form of increased fat infiltration or smaller total cross-sectional area, may contribute to LBP and poor function [5,6,7]. In this text, ‘degenerative lumbar spine disease’ is used to refer to this varied group of pathologies that may ultimately lead to segmental instability, radiculopathy, LBP and diminished function.
Current guidelines suggest that LBP without a specific cause should be managed conservatively through multidisciplinary rehabilitation and pharmacologic therapy where possible [8, 9]. Alternatively, in cases where conservative treatment has failed or in those with concurrent spinal stenosis or radiculopathy, surgical management may be considered [8]. At present, there is some evidence to suggest that changes in the morphology of multifidus following a surgical procedure are associated with worse clinical outcomes, although the magnitude of this effect is unclear [10,11,12,13,14]. Furthermore, the association between the pre-operative morphological condition of multifidus and post-operative clinical outcomes is not well described [15,16,17,18,19].
MRI is the preferred imaging modality to assess spinal pathology and guide suitability for surgical management. Additionally, MRI is a valid method of investigating multifidus musculature, with grading methods using either fat infiltration [20,21,22,23] or total cross-sectional area [24] to assess muscle quality. Therefore, pre-operative multifidus morphology on MRI may be a factor influencing clinical outcomes for those with degenerative lumbar spine disease.
The objective of this review is to investigate the association between pre-operative multifidus morphology on MRI and post-operative clinical outcome measures of LBP or disability after surgery, in adults with degenerative lumbar spine disease.
The protocol for this review was registered with the PROSPERO systematic review protocol registry (https://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42018090577).
The review included any published cohort studies and randomised control trials. Studies were included if they examined adults with acute or chronic lumbar spinal pain or radicular leg pain that were managed surgically for treatment of low back pain or radiculopathy. Studies were excluded if they examined asymptomatic adults or those with non-spinal lumbar back or leg pain, such as cancer-related back pain, pregnancy-related back pain, infection, inflammatory arthritis, stroke, cerebral palsy, or systemic neurological disease. Reviews, commentaries, conference abstracts were excluded.
The aim of this review was to identify the longitudinal association between pre-operative multifidus morphology and change in clinical outcome measures of low back pain or disability pre-operatively and at follow-up after surgical treatment. Clinical outcome measures included were pain (measured using a visual analogue scale, McGill pain questionnaire, or a self-efficacy questionnaire), disability (measured using the Oswestry disability index, the Roland-Morris disability questionnaire, a patient-specific functional scale, or equivalent index), work status (defined as return to work or school at follow-up) and health-related quality of life (measured using the euroQOL-5D or equivalent questionnaire).
We searched the Cochrane Library, EMBASE (1980 to January 2018), MEDLINE (1946 to January 2018), CINAHL (1937 to January 2018), Web of Science Core Collection (1900 to January 2018), and Scopus (1970 to January 2018) databases. There were no publication year restrictions.
The search was carried out on 24 January 2018, and included keywords such as “back pain”, “magnetic resonance imaging”, “paraspinal muscle”, “multifidus” and “therapeutics”. The search strategies for each database are detailed in our supplemental materials.
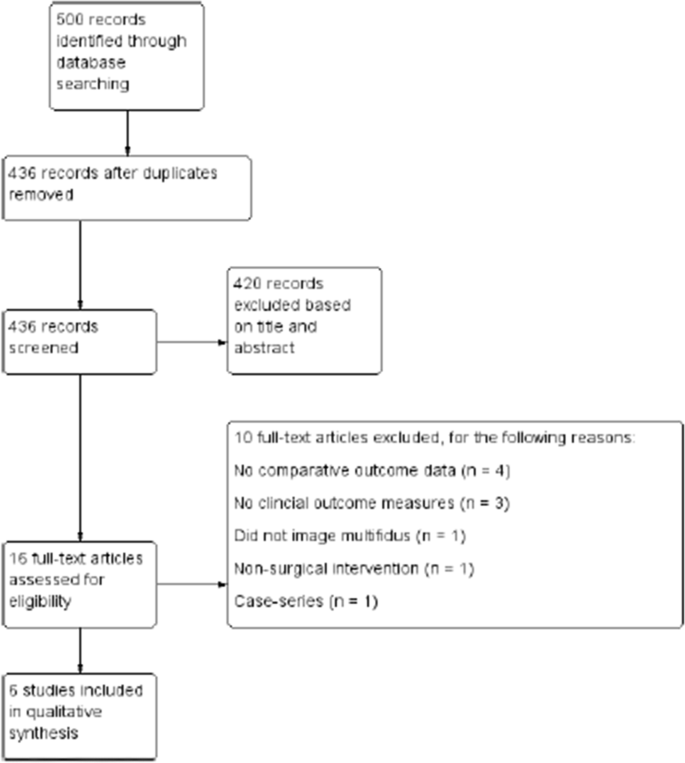
We screened titles and abstracts for relevant articles retrieved from the electronic search according to the eligibility criteria detailed above. Full-text articles that were considered relevant were obtained where possible and then assessed in the same manner. The study selection process is illustrated in Fig. 1.
Data were collected using a data extraction form adapted from the Cochrane Public Health Group Data Extraction Template [25]. These data were then entered into a word processing software package. For each paper, two authors completed the data extraction form independently. Disagreements were adjudicated by a third author.
The data extracted were: study design, method of recruitment, sampling technique, participant demographics, disease examined, intervention performed, method of multifidus morphology measure (cross-sectional area or fat infiltration on MRI) and pre- and post-operative clinical outcome measures.
Risk of bias was assessed using a modified version of the Quality In Prognosis Studies (QUIPS) tool [26], studies were rated as at high, moderate, or low risk of bias across six domains: (1) study participation, (2) study attrition, (3) measurement of multifidus morphology, (4) outcome measures, (5) study confounding and (6) statistical analysis and reporting. Overall risk of bias was determined based on the number of domains at moderate or high risk (Table 1). Two authors completed the risk of bias assessment independently, and any disagreements were adjudicated by a third author.Table 1 Method of assessing overall risk of bias using the QUIPS tool [26]Full size table
Level of fat infiltration was grouped as “low” (participants with no fat infiltration and mild fat infiltration) or “high” (participants with moderate and severe fat infiltration). An effect size (ES) was estimated using the standardised mean difference (SMD) for graphical illustration. For each study where a standardised mean difference could not be calculated, the effect size detailed in the individual study was reported.
A forest plot using a random effects model was generated for comparison between studies. A meta-analysis could not be performed due to significant heterogeneity between studies. Instead, a narrative synthesis was conducted.
Details of study selection are shown in Fig. 1. There were 500 titles retrieved from database searches, and 6 studies were included after screening and eligibility assessment. In total, six studies were included for data extraction and analysis.
Study characteristics are reported in Table 2. Of the six included studies, three were retrospective cohort studies [16,17,18], two were prospective cohort studies [15, 19], and one was a secondary analysis of a previous randomised control trial [13]. Two studies examined the effect of pre-operative fat infiltration on improvement in LBP (Visual Analogue Scale [VAS]) only [17, 18], two studies examined the effect of pre-operative fat infiltration on improvement in both LBP (VAS) and disability (Oswestry Disability Index [ODI]) [13, 15], one study examined the effect of both pre-operative fat infiltration and cross-sectional area on improvement in disability (ODI) only [19], and one study examined the effect of pre-operative cross-sectional area on improvement in disability (ODI) only [16]. No studies examined the effect of multifidus cross-sectional area on LBP rating scales. All included studies examined multifidus morphology using validated methods of imaging and grading; however, only two studies used identical methods of grading [17, 18]. For quantification of fat infiltration, two studies used pixel intensity to estimate grade of fat infiltration [17, 18] and three studies used a semi-quantitative visual method [13, 15, 19]. For measurement of cross-sectional area, both studies used manual tracing of muscle cross-sectional area from a digital image [16, 19].Table 2 Characteristics of included studiesFull size table
The results of the risk of bias assessment are reported in Table 3. Overall, two of the included studies were at high risk of bias [15, 16] and four were considered to be at low risk of bias [13, 17,18,19]. Generally, the QUIPS tool domains at greatest risk of bias were study confounding with three studies being at moderate-to-high risk of selection bias [15, 16, 18] and study attrition with three studies being at moderate-to-high risk of attrition bias [16,17,18].Table 3 Results of risk of bias assessment across the 6 domains of the QUIPS tool [26]Full size table
The six studies included in this review reported the results for 873 participants in total. Overall, all included studies reported a significant relationship between a measure of pre-operative high-quality multifidus muscle (i.e. low fat infiltration and/or greater total CSA) and greater improvement in at least one clinical outcome at follow-up. One study identified a significant relationship between pre-operative multifidus CSA, but not fat infiltration, and greater improvement in clinical outcomes [19]. The studies included all investigated the association between pre-operative multifidus morphology and post-operative clinical outcomes in different ways, and three different surgical interventions were performed across the six studies. As a result, it was not possible to aggregate the results in the form of a meta-analysis; thus, the data are presented as a narrative review.
An association between pre-operative multifidus morphology and change in pain at follow-up was apparent in all four studies investigating this relation, such that low fat infiltration was associated with a greater improvement in pain score when compared with moderate-to-high fat infiltration (Table 4) [13, 15, 17, 18]. Three of these studies were at a low risk of bias [13, 17, 18], and one was at a high risk of bias [15].Table 4 Studies examining pre-operative multifidus fat infiltration and painFull size table
Evidence for an association between multifidus fat infiltration and improvement in disability is conflicting (Table 5). Of the three studies investigating this relationship, one study at high risk of bias [15] and one study at low risk of bias [13] reported greater reductions in disability at follow-up in those with low fat infiltration compared to moderate-to-high fat infiltration pre-operatively.Table 5 Studies examining pre-operative multifidus fat infiltration and disabilityFull size table
There is limited evidence to suggest a greater multifidus cross-sectional area is associated with an improvement in disability at follow-up (Table 6). One high-risk study identified a moderate correlation between increasing CSA and lower post-operative ODI at follow-up [16], and one low-risk study identified those with a CSA > 8.5cm2 were more likely to report an improvement in ODI > 40% compared to those with a CSA < 8.5cm2 [19].Table 6 Studies investigating pre-operative multifidus cross-sectional area and disabilityFull size table
No studies investigated the association between pre-operative multifidus CSA and improvement in LBP at follow-up.
Three of the four studies examining the association between pre-operative multifidus fat infiltration and change in pain (VAS) reported sufficient data for comparison; however, a meta-analysis could not be performed due to significant heterogeneity between studies [13, 17, 18]. A forest plot without the pooled estimate is reported in Fig. 2.
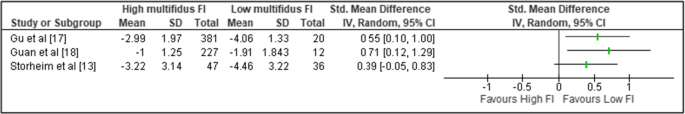
The findings of this review suggest a trend for better outcomes following surgery if pre-operative multifidus quality is higher. Although all studies reported improvements in pain and disability after surgery regardless of muscle quality, greater improvement was demonstrated with either low fat infiltration [13, 15, 17, 18] or large.
cross-sectional area [16, 19]. The strongest evidence was for patients with low multifidus fat infiltration having a greater reduction in LBP at follow-up when compared to patients with moderate-to-high fat infiltration [13, 15, 17, 18].
The evidence for low fat infiltration and post-operative reduction in disability is weaker, with only two out of three studies demonstrating a greater reduction compared to moderate-to-high fat infiltration [13, 15, 19]. Patients with larger multifidus CSA demonstrated greater improvement in disability; however, only two studies investigated this relationship [16, 19] and no evidence investigating multifidus CSA and post-operative reduction in LBP could be identified. As a result, the evidence for multifidus CSA as a prognostic factor is limited.
Outwith surgical treatment, both smaller CSA and greater level of fat infiltration in the multifidus muscle are associated with LBP, and reduced CSA is predictive of LBP up to 12-months [6, 7]. The ability of the multifidus muscle to stabilise and control movement of the lumbar spine may be a key factor in development of LBP, with evidence suggesting those with LBP have lower activity in multifidus [29]. Additionally, a rehabilitation approach focused on training multifidus can reduce LBP following injury [30]. As patients with poor quality multifidus muscle at diagnosis often experience more severe LBP and disability, it may be that superior multifidus morphology indicates a better starting point for surgery [5,6,7]. Indeed, three of the included studies reported patients with poor quality multifidus muscle had worse LBP and/or disability at baseline [13, 17, 18], with only one study explicitly adjusting for this during analysis [13].
Low physical activity is associated with poorer multifidus morphology [31], and it may be that LBP in degenerative lumbar spine disease associated with low-quality multifidus muscle is less easily correctable by surgery. It may also be that multifidus morphology is a marker of the likelihood that a patient will engage with post-operative physiotherapy and rehabilitation. Therefore, optimising pre-operatively multifidus morphology through targeted training may improve surgical outcomes. Furthermore, use of ultrasonography is a validated method of evaluating multifidus morphology and may be used as a faster, more cost-effective method of assessing multifidus morphology prior to surgical referral [32, 33].
The potential for muscular atrophy to be a predictor of surgical outcomes is not limited to spinal surgery. Fat infiltration and muscular atrophy of the shoulder rotator cuff on pre-operative MRI has also been found to correlate with worse post-operative outcomes following surgical repair compared to patients with higher quality rotator cuff musculature [27, 28].
Research on the predictive value of multifidus morphology is relatively new, and the volume of published literature is small. Thus, the possibility of publication bias could not be eliminated. Attempts to minimise this were made by making the literature search as complete as possible and including research in abstract-only format; however, accounting for results from the unpublished literature was not possible.
Additionally, the included studies were dissimilar in terms of fat infiltration grading technique, surgical intervention and reporting of outcomes for individual patients; thus, a summary statistic of effect size including all the available literature could not be calculated.
Ideally, future studies should report the timing of pre-operative pain score and pre-operative MRI, as well as the period of conservative management prior to surgery. In addition, controlling for greater LBP at baseline will help clarify the effect of poor multifidus morphology on surgical outcomes.
This systematic review found evidence for an association between low multifidus fat infiltration on MRI at baseline and greater reductions in measures of LBP and disability following surgical treatment. There is also limited evidence for an association between larger pre-operative multifidus CSA and improvements in disability, but not pain. The findings of this review should be interpreted with caution due to the small quantity of available literature.
This review highlights the theoretical potential of multifidus morphology as assessed by MRI to be an additional predictor of LBP and disability following surgery. As pre-operative MRI scanning is performed routinely on patients undergoing spinal surgery, it would require minimal change in clinical practice to implement, beyond use of a standardised method to quantify muscle quality. However, there is a need for more high-quality research elucidating this association before recommendations for clinical practice can be made.
Future research should address the methodological limitations of the currently published literature by more completely reporting the source of the sample population and reasons for participant drop-out, incorporating a prospective design, and controlling for potential confounding factors. Additionally, studies further examining the relationship between pre-operative multifidus CSA and improvements in LBP and disability at follow-up are required.
This study was unfunded.
Correspondence to Andreas K. Demetriades.
All authors confirm that they have no potential conflict of interest.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.