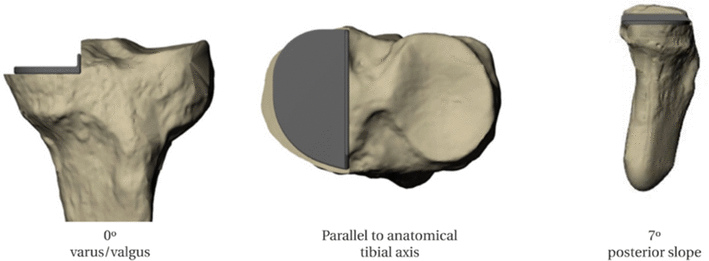
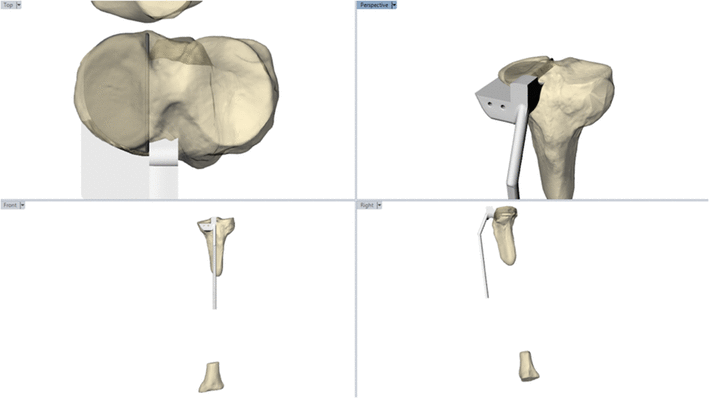
This study addressed one of the factors in the low uptake of UKA surgery, despite its many advantages to patients. Given the high degree of technical expertise required, we set out to determine whether assistive technology, in the form of low-cost 3D printed instruments, has the potential to improve surgical accuracy in low-volume surgeons.
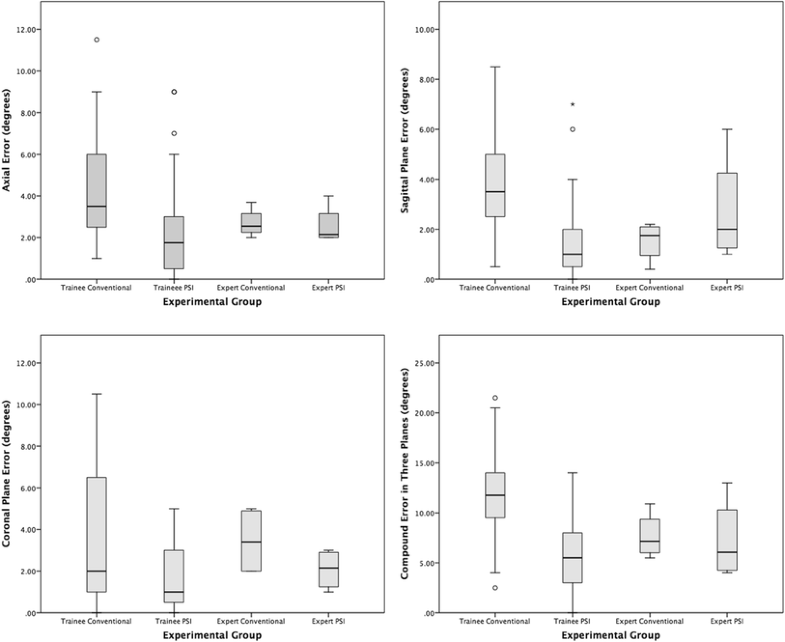
Unsurprisingly, this study shows that overall, using conventional instruments, expert surgeons are significantly more accurate than trainee surgeons at performing the saw cuts necessary to position a medial UKA tibial component according to a pre-operative plan. However, PSI immediately allowed the same trainee surgeons, who had not previously performed a UKA, to achieve the same level of overall accuracy as the expert surgeons. Furthermore, for coronal alignment PSI actually made the trainees, and experts, significantly more accurate than the experts using conventional instruments. Despite this, the PSI cutting guide had no impact on the overall accuracy of the expert surgeons.
The largest error for the expert group using conventional instruments was in the coronal plane, with no statistical difference between the experts and trainees, and significantly lower errors for both the trainees and experts using PSI. Indeed, all four conventional expert saw cuts resulted in more varus angulation than planned (2°–5°). Although this represents a failure to reproduce the pre-operative plan of a tibial component orthogonal to the tibial mechanical axis, it might reflect a subconscious desire to recreate the natural joint line obliquity, which is associated with a lower risk of UKA revision [19, 20]. This might have been exacerbated because the sawbone had a medial proximal tibial angle (MPTA) of 83°, which is outside the normal range [21].
Also interesting was the larger than expected, although not statistically significant, sagittal plane error for the expert group using PSI. This was due to a single large error of 6° by a surgeon unfamiliar with PSI, whose focus on fitting the guide to the bone meant that their normal routine of confirming extra-medullary alignment guide parallelity with the tibial shaft was overlooked. The same error befell the two outliers in the trainee PSI group, and is probably an example of inattentional blindness due to a focus on the new technology. This underlines the importance of simulation training before any technology is used in a clinical setting [22]. The latest Embody PSI design (Embody, London, UK) addresses the risk of sagittal plane error by incorporating a novel patient-specific ankle guide which fixes the posterior slope as per the pre-operative plan.
According to the manufacturer’s guidelines on acceptable implant position in the sagittal (± 5°) and coronal planes (± 5°), for the trainees PSI reduced the number of outliers from 16 to 1 [17]. For the experts, the single large error in posterior slope with PSI meant that the number of outliers increased from zero to one with PSI. These results are comparable to the three outliers reported by Kerens et al. [23] in their first 30 cases using Zimmer Biomet’s commercially available MRI-based Signature® PSI (Warsaw, Indiana).
Two randomised controlled trials (RCTs) concluded that, using 2D radiographs, that MRI-based PSI for UKA does not improve implant positioning compared to conventional instrumentation [14, 24]. However, these two RCTs were conducted by expert UKA surgeons in high-volume centres, so an alternative interpretation is that PSI allows surgeons to replicate expert results. Two previous studies have examined the role of PSI for inexperienced surgeons [25, 26]. A small sawbone study of 16 trainee surgeons, who performed lateral UKA using CT-based PSI and conventional instruments, found no difference in accuracy of implant alignment in the coronal, sagittal, or axial planes between the techniques [25]. However, a recently published clinical trial comparing 25 medial UKA performed using MRI-based PSI by a surgeon with no prior UKA experience, and 25 performed using conventional instrumentation by a surgeon ‘with wide experience’ of UKA, found no difference in tibial component alignment, patient reported outcome scores, or 2-year survival rates [26]. It should be noted that only coronal plane positioning was specified pre-operatively and unfortunately no detail was given regarding the training received by the one inexperienced UKA surgeon. Nonetheless, the results are encouraging and consistent with the findings in our study.
Other factors, such as patient selection, might contribute to the caseload effect on UKA revision rates. However, it is known that UKAs performed by low-volume surgeons are more likely to be revised for aseptic loosening and unexplained pain [9]. Evidence from previous studies suggest that tibial component positioning is an important factor in both these factors. In a retrospective review of 559 medial UKAs by Chatellard et al. [13], tibial components orientated more than 3° from the native joint line in the coronal plane were associated with decreased prosthesis survival. However, a finite element (FE) model analysis by Innocenti et al. predicted higher stresses in the underlying cancellous bone when the tibial implant was positioned outside of 0°–3° varus in the coronal plane [27]. As well as increasing the risk of aseptic loosening, higher bone stresses increase the risk of revision surgery due to pain [28]. In the sagittal plane, Chatellard et al.’s study also identified that a posterior slope greater than 5°, or a change in the native tibial slope of more than 2°, increased the risk of implant failure [13]. This is supported by biomechanical studies, with a significant increase in underlying bone strain with posterior slopes greater than 5° [29, 30]. In our study, this level of accuracy was delivered using PSI, with mean absolute error in tibial component positioning for the trainees being significantly less than 3° in the coronal and sagittal planes (p < 0.0001 for both). Yet, with conventional instrumentation, mean trainee error was not less than 3° in the coronal plane (p = 0.3382) and significantly more than 3° in the sagittal plane (p = 0.0259).
The cost-effectiveness of assistive technology is relevant to this study. A Markov model analysis of robotic-assisted UKA calculated an incremental cost of $150,000 per quality-adjusted life-year (QUALY) with a case volume of 32 per annum [31]. For lower-volume surgeons, who arguably have the most to gain from assistive technology, the price per QUALY would be even higher. Although PSI is undoubtedly cheaper than robotic technology, there are no published cost-effectiveness studies of PSI for UKA. In TKA, the value of PSI remains unclear; two studies have concluded that associated improvements in operating room efficiency offset any additional costs [32, 33], whilst a study by Barrack et al. [34] reached a different conclusion. However, these studies are based on the assumption that PSI does not improve component alignment in TKA, which our sawbone study suggests might not be the case for low-volume UKA surgeons.
Our study has some limitations. The results only apply to the two designs of guide tested, and therefore cannot be generalised. Oxford Phase III instruments (Zimmer Biomet, Bridgend, UK) instruments were used in this study. A newer instrumentation set (Oxford Microplasty) has been developed by the same manufacturer, but uses a similar instrument to guide tibial resection in the coronal, sagittal, and axial planes. Indeed, a recent study by Walker et al. [35] comparing their first 100 cases using the Phase III instruments, with their first 100 cases using the Microplasty instruments, found no difference in the accuracy of tibial component positioning. Trainees without prior experience of UKA were tested, and it is unknown whether their results are representative of low-volume UKA surgeons. Albeit, the most common mean UKA caseload in the UK is one per year, and so arguably these surgeons are not much more familiar with the procedure than a trainee. It is also important to recognise that the results probably represent a best case scenario for both instruments, because, in vivo, the visualisation of key landmarks through a mini-arthrotomy is more challenging, and soft tissue makes PSI positioning more difficult. It was assumed that the tibial implant sits perfectly on the cut bone surface, and so any potential variability between expert and trainee cementation technique was not considered. However, this would not be a factor when using the newer cementless Oxford implants (Zimmer Biomet, Bridgend, UK), which have been shown in an independent series to be a safe alternative to the cemented version [36]. The decision to examine tibial component alignment, but not resection depth, was a conscious one given that resection depth is an intra-operative decision based on soft tissue tension.