November 2019, Volume 28, Issue 11, pp 2579–2587|
Djuric, N., Yang, X., Ostelo, R.W.J.G. et al. Eur Spine J (2019) 28: 2579. https://doi.org/10.1007/s00586-019-06108-9
To study the interaction between Modic changes (MC) and inflammation by macrophages in the disc, in relation to clinical symptoms before and after discectomy for lumbar disc herniation.
Disc tissue was embedded in paraffin and stained with haematoxylin and CD68. Subsequently, tissue samples were categorized for degree of inflammation. Type of MC was scored on MRI at baseline. Roland Disability Questionnaire (RDQ) score and visual analogue scale for back pain and leg pain separately were considered at baseline and 1-year follow-up post-surgery. Main and interaction effects of MC and inflammation were tested against clinical outcome questionnaires. In addition, this analysis was repeated in bulging and extruded discs separately.
Disc material and MRI’s of 119 patients were retrieved and analysed. Forty-eight patients demonstrated mild inflammation, 45 showed moderate inflammation, and 26 showed considerable inflammation. In total, 49 out of 119 patients demonstrated MC. Grade of disc inflammation did not associate with the presence of MC. At baseline, no main or interaction effects of MC and inflammation were found on the clinical scores. However, during follow-up after discectomy, significant interaction effects were found for RDQ score: Only in patients with MC at baseline, patients remained significantly more disabled (3.2 points p = 0.006) if they showed considerable disc inflammation compared to patients with mild inflammation. The additional analysis showed similar results in extruded discs, but no significant effects in bulging discs.
An interaction effect of MC and disc inflammation by macrophages is present. Only in patients with MC, those with considerable inflammation recover less satisfactory during follow-up after surgery.
These slides can be retrieved under Electronic Supplementary Material.

Sciatica Inflammation Macrophage Roland disability questionnaire Modic type 2 changes
The online version of this article ( https://doi.org/10.1007/s00586-019-06108-9) contains supplementary material, which is available to authorized users.
Patients with lumbar disc herniation often suffer from radicular pain symptoms, the origin of which is not fully understood. Even though a part can be explained by mechanical compression of the nerve root, inflammation also seems to play a major role. Disc inflammation may occur if the nucleus pulposus herniates into the epidural space. This may induce a foreign-body reaction accompanied by neovascularization and macrophage infiltration [1].
Macrophages may subsequently induce a resorption process by excreting matrix metalloproteases [2], inducing apoptosis and degrading collagen fibres [3]. This so-called functional inflammation response can explain spontaneous regression in herniation size [4]. On the other hand, they can also excrete pro-inflammatory cytokines such as IL-6, IL8 and TNF-alpha, which have been associated with exacerbation of the pain symptoms, the so-called painful inflammation response [5, 6, 7]. This discrepancy in inflammation response is reflected in the inconsistent findings regarding the correlation between the presence of macrophages in herniated disc material and clinical symptoms [8, 9]. As of today, it remains unknown what factors are of influence on the type of inflammation response that patients experience. Nevertheless, the degree of inflammation can be influenced by specific characteristics of the disc. For example, extruded discs, which are more exposed to the systemic circulation, tend to have a higher degree of inflammation as compared to bulging discs [4]. Therefore, the type of disc herniation should not be neglected when the characteristics of disc inflammation are studied.
Modic changes (MC), also known as vertebral endplate signal changes (VESC) on MRI, are often seen in patients with lumbar disc herniation [10] and have been proposed to associate with slowing the recovery rate in patients that suffer from a herniated disc [11]. In addition, Dudli (2017) introduced the suggestion that MC represent the effect of crosstalk between bone marrow and the intervertebral disc [12]. Hence, changes in the vertebral endplate, like inflammatory dysmyelopoiesis and upregulation in neurotrophic factors, may induce the presence of different types of macrophages, which may interact with the inflammation response in the disc. By doing so, possibly, MC represent a shift in the above-mentioned inflammation reaction from ‘functional’ towards ‘painful’, thereby lowering the rate of recovery [11]. Until now, no studies have sought to enlighten this interaction. Therefore, the aim of the present study is to explore the interactions between disc inflammation and MC on the rate of recovery after surgery.
A significant interaction effect is expected between disc inflammation and MC on the clinical symptoms: Patients with MC will suffer from a high degree of inflammation, while patients without MC will benefit from inflammation. In addition, we expect this effect to be clearer in extruded discs as compared to bulging discs.
This retrospective study was performed using participants from the Sciatica Trial [13], a multicenter RCT with 283 patients who suffered from sciatica for 6–12 weeks and had a disc herniation as assessed by means of MRI. A total of 141 patients were randomized to surgery, and 125 patients actually underwent surgery (16 recovered before surgery could be performed). The other 142 patients were randomized to prolonged conservative care, of which 55 patients underwent surgery within 1 year, with a mean time to surgery of 15 weeks after randomization. Thus, in the first year after randomization, a total of 180 patients underwent surgery for sciatica. Out of the 180 patients, 120 disc samples were available for analysis. Missing samples were due to multiple reasons: not collected during surgery, got lost after surgery, not preserved properly, or got lost after preservation. All surgeries were performed between November 2002 and February 2005. The protocol, which included analysis of the disc material, was approved by the medical ethics committees at all participating hospitals.
Disc material of all operated patients was collected and fixed in 4% formaldehyde solution after surgery and was subsequently stored for future analysis. For the purpose of this retrospective study, samples were embedded in blocks of paraffin and stained with CD68 to evaluate inflammation by macrophages. A detailed description of the protocol was published in our previous work [14].
The evaluation was done by two independent investigators, who were trained by a senior pathologist and were blinded to clinical information. The number of macrophages in each sample was counted semiquantitatively and categorized according to their inflammation grade. The categories consisted of mild (0–10 macrophages per cm2), moderate (10–100 macrophages per cm2), and considerable (> 100 macrophages per cm2) inflammation. Subsequently, the inter-observer agreement between the independent researchers was determined. The inter-observer agreement value was predefined to be over 60%. Therefore, it was scheduled to reevaluate the tissue sample if the agreement was less than 60%, involving evaluation of the senior pathologist. After the consensus reading, a consensus score was calculated.
MRI scans were performed at baseline by a 1.5-Tesla scanner, and both sagittal T1- and T2-weighted images of the lumbar spine were evaluated. According to the criteria of Modic et al. [15, 16], Modic changes were scored as Type 1, Type 2, or Type 3. Type of disc herniation as quantified by MRI was categorized as follows: bulging, extrusion, sequestration or not applicable. The definition of protrusion as defined by the protocol is more commonly known as ‘bulging’ in daily clinical practice. Therefore, this term is used in this article. MRIs were evaluated by two neuroradiologists and one neurosurgeon. All three were blinded to histological data and clinical information. The readers were not involved in the selection or treatment of the patients included. Inter-observer agreement analysis was published earlier by el Barzouhi et al. [17]. Because in earlier findings no MC were observed at level L1-L2, only images from L2-L3 through L5-S1 were evaluated. For the statistical analyses, the majority opinion of the three independent researchers (answer by at least two of the three MRI assessors) was used.
The clinical outcome parameters from the Sciatica trial that we used to associate the histological data with were the Roland Disability Questionnaire (RDQ): the scores ranging from 0 to 23, with higher scores indicating worse functional status [18], and the 100-mm visual analogue scale (VAS) for leg and back pain: with 0 representing no pain and 100 the worst pain ever experienced [19]. These outcome measures were considered at baseline, and at 2, 4, 8, 12, 26, 38, 52 weeks post-surgery. For the RDQ score, a difference of 3 points was regarded as the minimal clinically important change [20]. For the VAS score, this threshold was set at 19 points [21].
First, the presence of MC was tested against the dichotomized histological findings and against the type of disc herniation using Chi-square tests for categorical data. The association between disc type and inflammation was previously reported [4]. For the clinical analysis at both baseline and follow-up, patients were first grouped based on the type of herniation, Subsequently, the main effects of inflammation and MC, and their interaction effect were tested against the clinical baseline scores (RDQ, VAS leg pain and VAS back pain). For this analysis, a two-way ANOVA was used.
In order to analyse the predictive value of inflammation and MC at baseline, these were tested for associations with clinical outcomes (VAS back, VAS leg and RDQ) over the course of 1-year follow-up using a mixed model analysis. The clinical outcomes were measured at 2, 4, 8 12, 26, 36 and 52 weeks post-surgery. For both baseline and follow-up analyses, Bonferroni correction for multiple testing was used. If additional post hoc tests were indicated, those were corrected for the number of post hoc tests. P values of < 0.05 were regarded as significant.
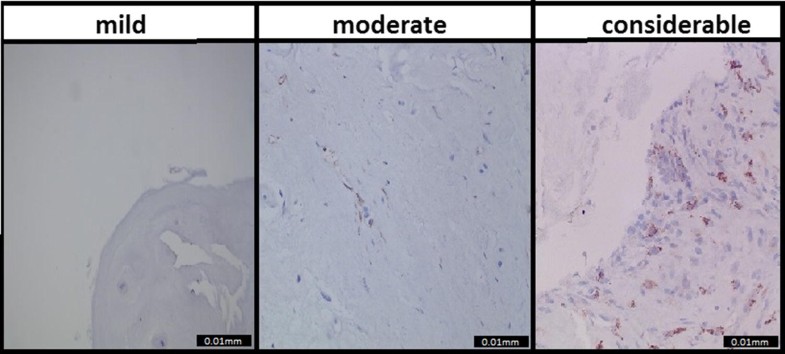
CD68 staining to identify macrophages resulted in the following distribution: 48 (40%) patients were scored as mild inflammation, 45 (37.5%) patients as moderate inflammation, and 27 (22.5%) patients as considerable inflammation. The consensus score was excellent (0.96) [6]. Examples of the CD68 samples and their categories are shown in Fig. 1.
Of the 120 patients included, one patient’s MRI was lost, two MRI’s showed MC type 1 changes (MC1) and 47 showed MC type 2 (MC2), of which 42 at the level of the disc herniation, and 70 showed no MC (inter-observer agreement 69–97%). Because only two patients demonstrated MC1, this subgroup was not suitable for statistical analysis. Hence, they were excluded from the analyses. Regarding disc type, 40 discs were characterized as bulging disc and 73 as extruded disc, no sequestrations were seen (kappa = 0.62).
Baseline characteristics age, gender, BMI and duration of sciatica symptoms prior to surgery in the three inflammation groups were comparable (Table 1). After surgery, VAS leg and back pain and RDQ scores decreased significantly in all patients.Table 1
Baseline characteristics of the three histologically defined inflammation groups for patients with and without Modic type 2 changes
| No/mild (without MC) (N = 31) | No/mild (with MC) (N = 16) | Moderate (without MC) (N = 25) | Moderate(with MC) (N = 19) | Strong(without MC) (N = 14) | Strong(With MC) (N = 12) | |
|---|---|---|---|---|---|---|
| Age | 39.0 ± 8.9 | 43.1 ± 10.7 | 39.8 ± 9.4 | 45.1 ± 11.1 | 40.7 ± 6.5 | 47.8 ± 4.4 |
| Male (%) | 83.9% | 62.5% | 52% | 73.7% | 71.4% | 100% |
| Body mass index | 26.0 ± 4.3 | 26.3 ± 3.7 | 25.2 ± 3.8 | 26.0 ± 2.7 | 24.2 ± 2.3 | 27.6 ± 3.0 |
| Duration of sciatica in weeks | 9.4 ± 1.8 | 10.0 ± 2.2 | 9.5 ± 2.5 | 9.8 ± 2.3 | 8.6 ± 2.3 | 8.5 ± 1.9 |
| Roland Disability score | 16.5 ± 4.8 | 16.2 ± 3.6 | 17.0 ± 4.2 | 17.3 ± 3.4 | 16.6 ± 4.3 | 16.5 ± 5.1 |
| VAS leg pain | 64.6 ± 22.5 | 63.0 ± 20.6 | 69.9 ± 16.7 | 61.1 ± 25.2 | 76.4 ± 10.4 | 67.3 ± 22.1 |
| VAS back pain | 27.8 ± 31.7 | 46.6 ± 32.8 | 40.5 ± 27.4 | 28.3 ± 27.8 | 32.8 ± 28.0 | 21.3 ± 30.8 |
From one of the patients no data on Modic changes at baseline could be retrieved, Therefore the group of patients with ‘considerable inflammation’ is decreased to 26 patients. Values are presented as mean ± SD (except for the male/female ratio)
At baseline, no significant association was found between the grade of inflammation and the presence of MC2 (p = 0.525), nor between MC2 and disc type (p = 0.239) (Table 2). As described earlier, a positive association was found between bulging discs and the degree of inflammation [4].Table 2
Associations between MC and inflammation at baseline
| X2 test | No MC | MC2 | P value |
|---|---|---|---|
| Inflammation | 0.525 | ||
| Mild (n = 48) | 31 | 16 | |
| Moderate (n = 45) | 25 | 19 | |
| Considerable (n = 26) | 14 | 12 | |
| Type of herniation | 0324 | ||
| Not applicable (3) | 1 | 2 | |
| Bulging (40) | 27 | 13 | |
| Extrusion (73) | 41 | 32 | |
| Sequestration | 0 | 0 |
Values are n. P values of the Chi-square test are givenNo significant main or interaction effects were found between MC2 and inflammation by macrophages with either RDQ, nor with VAS leg pain nor with VAS back pain scores at baseline (Table 3). These results did not change when bulging and extruded discs were considered separately (Table S1).Table 3
Baseline main and interaction effects of MC2 and inflammation on clinical outcomes
| Mixed model test (patients with Modic changes): | RDQ core F value (p value) | VAS leg score F value (p value) | VAS back score F value (p value) |
|---|---|---|---|
| MC2 effect | 0.01 | 2.61 | 0.78 |
| Inflammation effect | (p = 1.000) | (p = 1.000) | (p = 1.000) |
| Interaction effect MC2* inflammation | 0.34 (p = 1.000)0.07 (p = 1.000) | 1.25 (p = 1.000)0.415 (p = 1.000) | 0.40 (p = 1.000)3.45 (p = 0.315) |
Two-way ANOVA was used, values are F values with p values, n total = 119. P values are corrected for multiple testing according to Bonferroni (9 tests)
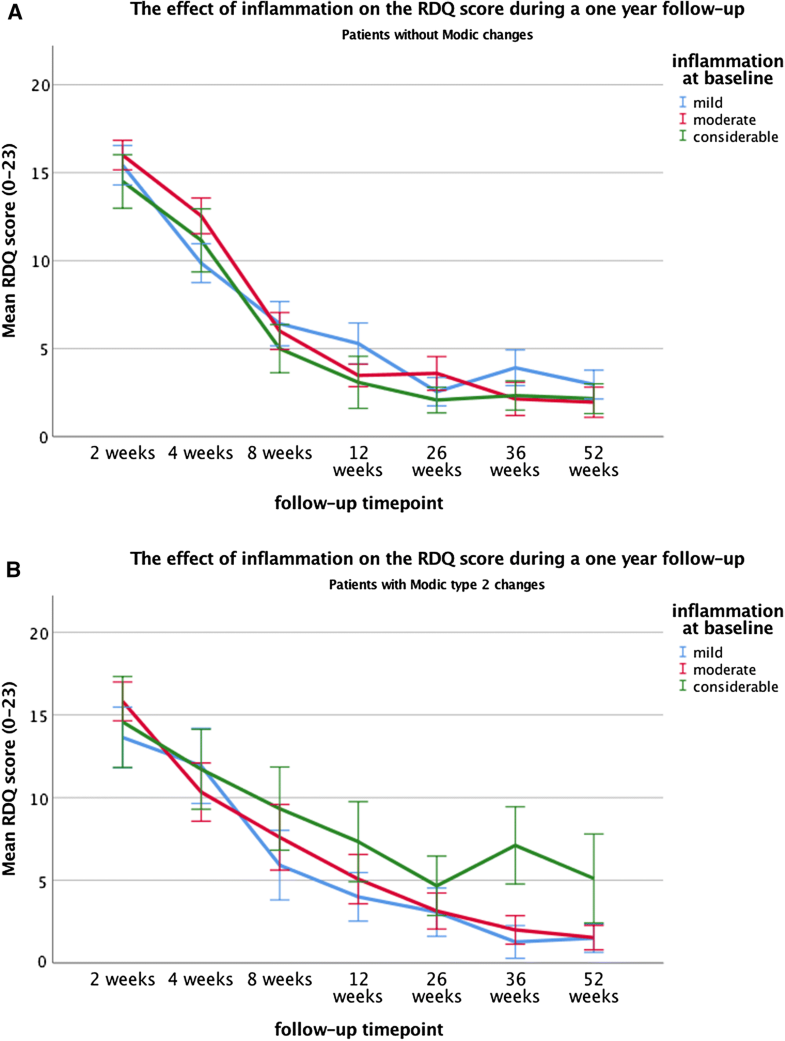
Clinical outcome during follow-up was measured at the time points 2, 4, 8, 12, 26, 38 and 52 weeks after surgery. However, the timing of surgery was not equal for all 117 patients. In the sciatica RCT, about 40% of patients randomized to conservative treatment, crossed over to surgical treatment because of unbearable symptoms. These patients were clinically evaluated at the same time points after randomization. However, surgical intervention was performed with a mean of delay 15 weeks, ranging from 15 days to 18 months after randomization. Mean waiting time prior to randomization was 9.4 weeks. In our sample group, 34 of 120 patients were originally randomized to conservative care and crossed over. The measurements at time points 2, 4, 8, 12, 26, 38 and 52 after randomization were likely influenced by surgical intervention effects. Therefore, patients with a deviation of more than 3 SD’s ‘delay to surgery time’ were considered outliers and excluded from the analysis. Twenty-one of the 34 patients were therefore excluded; 13 patients that remained had a mean waiting time for surgery of 25 days with a range of 2–51. The clinical data of the time points before the surgical intervention were ignored.Over the course of 1-year follow-up after surgery, no significant main effects were found of inflammation or MC2 on the RDQ score. However, a significant interaction effect was present (p = 0.009), indicating that the association between inflammation and the RDQ score depended on the presence or absence of MC2. Post hoc tests revealed that in the patient subgroup without MC, no significant associations between inflammation and RDQ score were found. On the contrary, in patients with MC, those with a considerable inflammation had significantly and clinically relevant higher RDQ scores (mean = 6.8) compared to those with moderate- (mean = 3.8, p = 0.019) or mild inflammation (mean = 3.6, p = 0.009) (Tables 4, 5, Fig. 2a, b).Table 4
Follow-up main and interaction effects of MC2 and inflammation on clinical outcome
| Mixed model test (patients with Modic changes): | RDQ score F value (p value) | VAS leg score F value (p value) | VAS back score F value (p value) |
|---|---|---|---|
| MC2 effect | 2.47 | 0.02 | 1.52 |
| Inflammation effect | (p = 0.963)2.25 (p = 1.000) | (p = 1.000) | (p = 1.000) |
| Interaction effect MC2* inflammation | 7.10 (p = 0.009) | 6.97 (p = 0.009)4.69 (p = 0.090) | 2.32 (p = 0.891)0.75 (p = 1.000) |
Mixed model analysis was used, values are F values with p values, n total = 96. P values are corrected for multiple testing according to Bonferroni (9 tests)Table 5
Post-hoc test for the main effects of inflammation on the VAS leg score and interaction effects on the RDQ and VAS leg score during the 1-year follow-up
| Inflammation in the entire population | Mild (N = 46) | Moderate (N = 44) | Considerable (N = 27) | Mild versus moderate | Mild versus considerable | Moderate versus considerable |
|---|---|---|---|---|---|---|
| VAS leg | 14.1 (11.7; 16.4) | 7.8 (5.5;10.2) | 9.7 (6.7; 12.7) | p = 0.004 | p = 1.000 | p = 0.390 |
| Inflammation in patients without MC | Mild(N = 31) | Moderate(N = 25) | Considerable(N = 14) | Mild versus moderate | Mild versus considerable | Moderate versus considerable |
|---|---|---|---|---|---|---|
| RDQ | 4.5 (3.7; 5.4) | 4.0 (3.2; 4.9) | 3.4 (2.3; 4.6) | p = 1.000 | p = 1.000 | p = 1.000 |
| VAS leg | 16.7 (13.9; 19.5) | 8.2 (5.1;11.1) | 6.4 (2.6;10.3) | p < 0.001 | p < 0.001 | p = 1.000 |
| Inflammation in patients with MC2 | Mild (N = 15) | Moderate (N = 19) | Considerable (N = 12) | Mild versus moderate | Mild versus considerable | Moderate versus considerable |
|---|---|---|---|---|---|---|
| RDQ | 3.6 (2.4; 4.8) | 3.8 (2.7; 5.0) | 6.8 (5.4; 8.1) | p = 1.000 | p = 0.009 | p = 0.019 |
| VAS leg | 11.4 (7.6; 15.2) | 7.5 (3.7; 11.2) | 13.1 (8.4; 17.7) | p = 1.000 | p = 1.000 | p = 0.975 |
Which subgroups explain the main/interaction effect found in Table 4. mean RDQ and VAS leg scores are compared between different degrees of inflammation, separately for patients in the entire population (n = 97) and with and without MC2 (n = 96). Values are means (Confidence Intervals), and p values, which are corrected for multiple testing according to Bonferroni (15 tests)
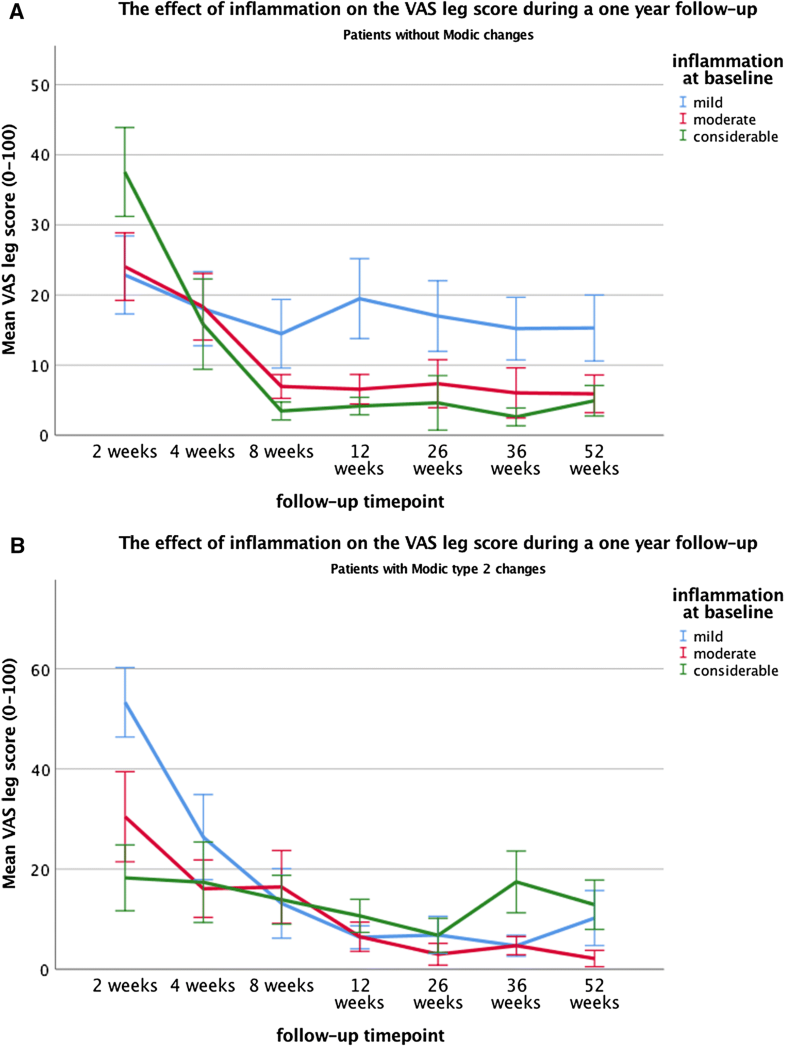
Furthermore, a significant association was found between inflammation and the VAS leg pain score during follow-up (p = 0.009) (Tables 4, 5). However, when assessing the interaction effect between MC2 and inflammation, this association between inflammation on the VAS leg score was only seen the patient subgroup without MC, in which the patients with mild inflammation experienced significantly higher VAS leg score (mean = 16.7) compared to both the moderate (mean = 8.2, p < 0.001) and considerable inflammation (mean = 6.4, p < 0.001). In contrast, no significant associations between inflammation and VAS leg scores were found in the subgroup of patients with MC2 (Table 5, Fig. 3a, b). Even though these associations were significant, they did not exceed the clinically relevant threshold of 19 points [21]. Moreover, no significant main effect of MC2 was found on VAS leg score.
At last, no significant associations were found between inflammation and VAS back scores or between MC2 and VAS back scores. Also, no interaction effects were found, indicating that the no associations between inflammation and VAS back score were seen in both patients with and without MC2 (Table 4).
When bulging discs and extruded discs were considered separately, extruded discs illustrated similar significant interaction effects between MC2 and inflammation on the RDQ (p = 0,003) and VAS leg score (P = 0,05) (Table S1), post hoc tests also revealed similar mean differences (Table S2). In contrast, no significant effects or interactions were seen in bulging discs.
The present study demonstrates that the association between inflammation, as identified through macrophage infiltration, and clinical outcome after lumbar discectomy depends on the presence or absence of MC2. Patients with MC2 and considerable inflammation recovered less satisfactory after surgery in terms of disability compared to MC2 patients with mild inflammation. These results were not seen in patients without MC. In addition, in patients without MC, leg pain decreased slightly more in those with considerable inflammation as compared to those with mild inflammation. This association was not seen in patients with MC2. Interestingly, when these bulging and extruded discs were considered separately, the above-mentioned results were only seen in extruded discs; in bulging discs, no significant effects were found. These results were in line with our hypothesis.
Here, inflammation was characterized by the presence of macrophages identified in disc tissue. As mentioned in the introduction, the type of macrophage responses can be characterized as either a ‘painful inflammation response’ or a ‘functional inflammation response’. This contradictory effect is likely due to the difference in macrophage differentiation; M1 macrophages will act pro-inflammatory, while M2 macrophages are involved in resorption [5]. Based on our results, we expect a high percentage of M2 macrophages in patients without MC. In contrast, a high percentage of M1 macrophages can be expected in patients with MC2. The alternative differentiation of macrophages could possibly be explained due to the exposure to outside factors. In patients without MC, this will be limited to macrophage infiltration from the epidural space, but in patients with MC, the disc will likely also be exposed to inflammatory factors excreted from the endplate [12]. Markers such as CSF1, CCL2, IL-1β and IL6 could subsequently lead to the macrophage differentiation towards M1 [5, 12]. In addition, since more macrophages are present in extruded discs, any interaction with the endplate excretion products will be more noticeable, which is illustrated by the fact that the studied effects were most prominent in discs with considerable inflammation. This may also explain the absence of significant effects in bulging discs, where considerable inflammation was very scarce and could thus not be analysed. Taken together, this insinuates that a threshold value of inflammation has to be reached before the interaction with MC2 becomes clinically relevant, which is unlikely occur in bulging discs, since they are less exposed to the epidural space. Nevertheless, it should be noted that the number of bulging discs in this study was low, and hence, the absence of effects in bulging discs could also be due to a limited number of samples in this subgroup.
An additional factor that may lead to macrophage differentiation towards M1 might be a bacterial infection of Propionibacterium Acnes, which has been associated with MC in disc herniation patients [22]. Inferences have been made that this underlying infection may cause the difference in inflammation profile [23], and thus for macrophage differentiation towards M1. Moreover, when Propionibacterium acnes is phagocytized by a macrophage, the bacteria can disrupt its lysosomal activity and remain latent for an extended period of time [24]. P. Acnes has also been known for creating a biofilm, which does not only increase the difficulty of detecting the bacteria, but also protects them from both host immune defenses and antibiotics treatments [25]. This increased tolerance to antibiotics could also explain how P. acnes can survive perioperative antibiotic prophylaxis. Taken together, an underlying infection of P. acnes could explain both the chronic inflammation process seen on MRI as MC, and the pathological effect that macrophage infiltration can have on recovery after surgery [26]. Unfortunately, studying the different types of macrophages or bacterial presence was beyond the scope of this study.
This study has some limitations: Samples were preserved in formaldehyde for many years, which may have reduced the quality. Nevertheless, the CD68 staining showed clearly identifiable macrophages, indicating that it was not a significant issue. Furthermore, only the number of macrophages and not T or B cells were used to study inflammation. This can be justified by previous findings, which identified macrophages as the main type of inflammatory cells in discus samples [8, 27], which makes them a reliable indicator of inflammation. At last, this study did not perform MRI scans with fat-saturated T2 sequences, which are the most sensitive for detecting bone marrow oedema and thus MC1 [28]. Hence, the found percentage of MC1 may be underestimated.
More importantly, in future studies, the dynamics of M1 and M2 macrophages in relation to bacterial infection of the disc and MC should be explored. Better understanding these may lead to a more accurate diagnosis, prognosis and treatment.
The authors thank Stefan Hoyng for his training of the researchers in cell-counting, and Ingrid Hegeman and Annemarie Sinke for the preparation and staining of the samples.
None of the authors has any conflict of interest. No funding was received for the conductance of this study.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
586_2019_6108_MOESM1_ESM.docx (105 kb)Supplementary material 1 (DOCX 104 kb)586_2019_6108_MOESM2_ESM.pptx (136 kb)Supplementary material 2 (PPTX 137 kb)
© The Author(s) 2019
Open AccessThis article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
CrossMarkCite this article as:Djuric, N., Yang, X., Ostelo, R.W.J.G. et al. Eur Spine J (2019) 28: 2579. https://doi.org/10.1007/s00586-019-06108-9