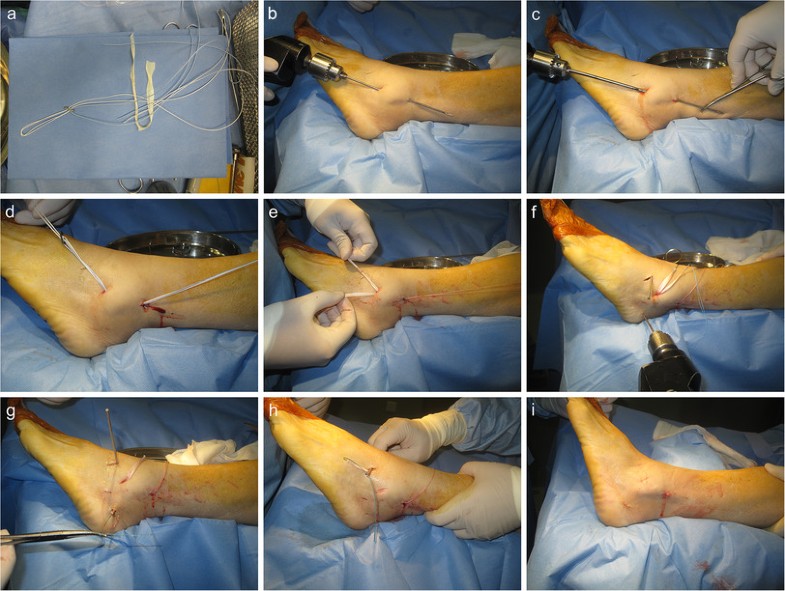
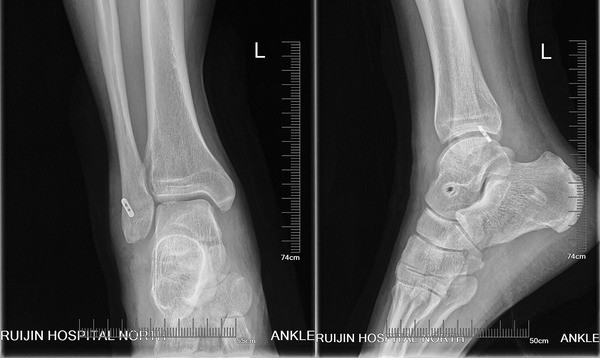
In this study, the patients achieved satisfactory clinical results after lateral ankle reconstruction using a percutaneous anatomic reconstruction technique with a Tightrope system. Our reconstruction method restored the normal anatomy by positioning the allograft at the original point ligament origin and insertion. There are two patients reported a residual instability on uneven ground, but they thought it was better than the preoperative condition. This study supports the effectiveness of this approach in this group of patients with severe instability.
To date, many surgical techniques have been described to manage CAI. These techniques and their modifications fall into three categories: non-anatomic reconstruction, anatomic repairment, and anatomic reconstruction. Non-anatomic reconstruction uses various configurations of local tendon grafts to accomplish the restriction function of the ligament without repair of the injured ligaments. Several techniques have been described, including partial or complete tenodesis from the peroneal tendon or Achilles tendon; or allografts mimicking the function of the original ligament such as the Chrisman–Snook (CS) [4], the Evans procedure [5] and the Watson Jones procedure [6]. Anatomic repairment is to restore normal anatomy and joint mechanics by in situ repair of the injured ligament. Anatomic repairment includes repair ligaments by either shortening and reattaching them to the bony surfaces, or augmenting them with surrounding structures to enhance the repairment. A good example is the classic Brostrom–Gould procedure [7], which empowers the original ligaments with the extensor retinaculum and has proved to be a strong procedure without sacrificing other anatomic structures. Anatomic reconstruction procedures use tendon grafts to recreate joint biomechanics anatomically by replicating the anatomic positions of the ATFL and CFL origin and insertion sites. They vary in the means by which they attain that positioning, including the number and angle of tunnels in the fibula and the fixation techniques selected in each bone tunnel location.
Non-anatomic techniques have been used in the past, but currently are not the procedure of choice, as such procedures do not reestablish the ankle kinematics, but stabilize the ankle and results in ankle stiffness [8, 9]. Now Brostrom–Gould procedure is considered to be the gold standard for surgical treatment of CAI [7, 10, 11, 12, 13]. However, anatomic repairment does not fully address special conditions such as severe instability or revision surgery. This procedure may not provide adequate stability and lead to recurrence using the weakened and scarred remnants. Subsequently, researchers have described several anatomic reconstruction procedures using autograft or allograft tendon [14, 15]. Studies have demonstrated that anatomic reconstruction can improve lateral ankle instability and restore normal ankle motion [16, 17, 18, 19]. Besides, there has been a recent trend of minimally invasive anatomic reconstruction of the lateral ankle ligaments for CAI, which has been found both feasible and reproducible. However, there is still large room to improve this technically demanding procedure.
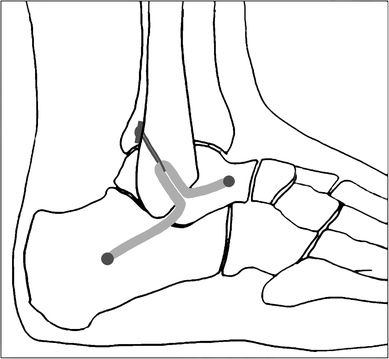
Some researchers, including our team, have reported a few minimally invasive techniques to reconstruct lateral ankle ligament. However, these strategies may not always follow accurate ligament anatomic attachments, especially in the construction of the fibular bone tunnels. Panchbhavi [19], Kim et al. [20] and Youn et al. [21] made a straight fibular tunnel in an anterior to posterior or otherwise direction, while Xu and Wang et al. [3, 22] made a ‘Γ’ shaped fibular tunnel.
To perform an anatomic reconstruction, the anatomy must be well understood. When performing an anatomic reconstruction of the lateral ligament complex, the surgeon has little guidance on where to place bony tunnels. Based on the research of eight unpaired fresh-frozen cadaver feet, Neuschwander et al. [2] demonstrated that the CFL and ATFL have a single confluent footprint on the anterior border of the distal fibula. Wenny et al. [1] also found that the fibular attachment of the CFL was suited direct adjacent to the fibular attachment of the ATFL. Therefore, these so-called anatomic reconstruction procedures could not fix the graft tendon at the original attachment point of ATFL and CFL anatomically. The reconstructed ligament in non-anatomic location will certainly have some effect on ankle rotational kinematics and kinetics during normal gait [23, 24]. In our study, we restored the ATFL and CFL anatomically from one common fibular origin, which better mimic the biological function of primary ligaments and should have resulted in more normal ankle kinematics. Besides, the graft in anatomic location is much likely to reduce soft tissue impingement and friction with lateral malleolus, articular surface of the talus, or peroneal tendon.
This procedure has several other advantages besides accurate anatomic localization. Creating a straight fibular tunnel is easier than previous ‘Γ’ shaped fibular tunnel [3, 22]. Furthermore, it spends less time with less intraoperative tunnel fracture probability. Two branch with a conjunct fibular outlet using Tightrope fixation will also reduce the risk of micromotion of the graft within the unfixed fibular tunnel, compared to previous bidirectional outlets that might have resulted in adjacent synovitis due to impingement or wearing of the graft. The traditional open techniques with larger incision put this anatomic region at a higher risk for colonization with microorganisms, nerve injury, ankle stiffness, and potential problems with wound healing than minimally invasive surgeries. This percutaneous technique has some merits for lateral ankle ligament reconstruction because it can access the same anatomic structures as an extensible approach without increasing the morbidity. Limited exposure reduces the likelihood of damage to the superficial peroneal nerve. The small incisions also have cosmetic appeal to young patients. It can be safely combined with arthroscopy as it preserves tissue structure, and allows early rehabilitation with less swelling and pain. Some argue that the real advantage of an open approach lies in simultaneous access to adjacent bones and tendons. Thus, in cases without these combined lesions, the open approach has no distinctive benefits. While compared to arthroscopic reconstruction, without complex threading and knotting, percutaneous method is easily reproducible, timesaving, and its learning curve is rapid. Meanwhile, it has the advantage of not preventing the use of arthroscopic methods, or open methods, in the case of failure.
Fixation using the Tightrope in our study is a relatively new technique compared with traditional methods. The Tightrope device was commonly used to offer cortical fixation for cruciate ligament reconstruction [25, 26, 27]. This suture-button system can facilitate folded graft fill of fibular tunnel and offer strong pullout strength. However, this system is not without its own problems. In our study, two patients reported soft tissue irritation from the cortical button. No granuloma formation or osteolysis in adjacent bone occurred in the patients.
This study had several limitations. First, the follow-up time was relatively short. The outcomes in this study are limited to the early results of treatment of CAI with a Tightrope system. We will continue to follow-up these patients. Second, the sample size was small. We are working on treating more patients with this procedure.